Official Collaborated with:


Speaker: Prof. Jay Shendure, Investigator, Howard Hughes Medical Institute Professor, Genome Sciences, University of Washington
Topic: Systematic Reconstruction of Mammalian Development
Time: 10 AM (HKT)
Zoom: Here
CTSCB Stem Cell Seminar Series
Date: July 23, 2024
Abstract: I will talk about our ongoing efforts to develop and apply scalable molecular profiling and molecular recording technologies to systematically reconstruct the entirety of mouse development at single cell resolution.
Biography: Jay Shendure, MD, PhD is an Investigator of the Howard Hughes Medical Institute, a Professor of Genome Sciences at the University of Washington, and Scientific Director of the Seattle Hub for Synthetic Biology (Allen-CZI-UW), the Allen Discovery Center for Cell Lineage Tracing, and the Brotman Baty Institute for Precision Medicine. His 2005 doctoral thesis with George Church included one of the first successful reductions to practice of next-generation DNA sequencing. Dr. Shendure's research group in Seattle pioneered exome sequencing and its earliest applications to gene discovery for Mendelian disorders and autism; cell-free DNA diagnostics for cancer and reproductive medicine; massively parallel reporter assays, saturation genome editing; combinatorial single cell molecular technologies; and genome editing-based molecular recording technologies. Dr. Shendure is the recipient of the Curt Stern Award from the American Society of Human Genetics (2012), the Richard Lounsbery Award from the National Academy of Sciences (2019) and the Mendel Award from the European Society of Human Genetics (2022). He is an elected member of the American Association for the Advancement of Science and the National Academy of Sciences. He serves or previously served as an advisor to the NIH Director, the US Precision Medicine Initiative, National Human Genome Research Institute, Chan Zuckerberg Initiative, Gladstone Institute, New York Genome Center and Allen Institute. He received his MD and PhD degrees from Harvard Medical School in 2007.

Speaker: Dr. Jun Wu, Associate Professor, Department of Molecular Biology, UT Southwestern Medical Center, Dallas, TX
Topic: Curious Cases of Pluripotent Stem Cell Adaptations
Time: 2:30 PM - 3:45 PM (HKT)
Venue: BSC Co-Working Area, 7/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: In-person at HK Science Park & Online via Zoom
CTSCB Invited Seminar by Dr. Jun Wu
Date: July 4, 2024
Abstract: Pluripotent stem cells (PSCs) harbor the extraordinary ability to self-renew and differentiate into nearly any cell type within an adult body. Over recent decades, the field of PSC research has experienced a renaissance of technological innovation that continually stretches the horizons of what's possible. Such advancements not only propel our understanding of mammalian development forward but also hold the promise to redefine regenerative medicine. PSCs are distinguished not just by their generative potential but by their unparalleled plasticity, allowing them to navigate and adapt to "foreign" environments in ways that challenge their inherent biological limits. These adaptations, although artificial in nature, provide windows into the molecular, cellular, and developmental principles of mammalian biology that remain elusive to traditional scientific inquiry. In my presentation, I will delve into some intriguing instances of PSC adaptations. Through these "curious" adaptations, we can develop novel tools and methodologies, broadening our understanding of the natural world in ways previously unimagined.
Biography: Dr. Jun Wu is an associate professor in the department of molecular biology at UT southwestern medical center. Dr. Wu’s work has contributed to the development of novel culture systems and methods that enable the generation of new stem cells for basic and translational studies. Dr. Wu has expanded the spectrum of pluripotent states by capturing mouse pluripotent stem cells (PSCs) with distinct molecular and phenotypic features from different developmental stages. And some of these culture conditions developed in mice enabled the generation of PSCs from many other mammalian species, including humans, non-human primates, and ungulates. In addition, Dr. Wu has developed an efficient and versatile blastocyst complementation system for in vivo generation of functional tissues and organs from cultured PSCs, and several stem cell-derived embryo models. Dr. Wu has received several awards including UT southwestern endowed scholar, CPRIT scholar, NYSCF-Robertson Stem Cell Investigator award, and 2024 ISSCR Outstanding Young Investigator Award.

Speaker: Dr. Hui Zhang, Principal Investigator, State Key Laboratory of Cardiovascular Disease, Beijing, China
Topic: Spatial Transcriptomics Explores Organ-Level Protection Strategies for the Heart
Time: 11:30 AM – 12:45 PM (HKT)
Venue: INNO², 2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: In-person at HK Science Park & Online via Zoom
CTSCB Invited Seminar
Date: June 26, 2024
Abstract: Drawing on the principles of "systems theory," our research seeks to alternative approach, or "leverage solution," to improve heart function after a heart attack, an area where traditional focus on direct heart repair has limitations. By using advanced techniques like single-cell and spatial transcriptome analysis, we explored various models in both mice and humans to pinpoint this "leverage solution" for repairing the heart. Our findings offer clear molecular proof for a new way the heart responds to injury at an organ level, leading to the development of innovative treatments to protect heart function from cardiac injuries.
Biography: Dr. Zhang Hui is a Principal Investigator at the State Key Laboratory of Cardiovascular Disease in Beijing, China. She integrates stem cell biology with epigenetic regulation, building on her education at East China Normal University,Shanghai and postdoctoral work at the University of Michigan Medical School, Ann Arbor. Her laboratory focuses on: 1) Employing multiomics data to uncover new therapeutic targets for cardiovascular diseases, 2) Creating human cardiac organoids for disease modeling and pre-IND drug assessment, and 3) Developing high-throughput methods for evaluating organoid functionality in assay development. Dr. Zhang's work innovatively contributes to drug discovery and the advancement of cardiovascular regenerative medicine through human cardiac organoid technologies.

Speaker: Prof. Cantas Alev, Institute for the Advanced Study of Human Biology (ASHBi), Kyoto University, Kyoto, Japan
Topic: Towards Reconstituting Human Axial Development In Vitro
Time: 11:00 AM – 12:15 PM (HKT)
Venue: BSC Co-Working Area, 7/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: In-person at HK Science Park & Online via Zoom
Registration: Here
CTSCB Invited Seminar
Date: June 17, 2024
Abstract: Recent efforts to reconstruct complex developmental processes in vitro from stem cells open up exciting new opportunities for studying the biological principles, which govern the emergence of form and function during embryonic development in humans and other non-model organisms. Early embryonic developmental events including somitogenesis, during which the metameric body plan of vertebrates is laid out, have been extensively studied using model organisms such as mouse or chick but remain largely elusive and poorly understood when it comes to human and other primates. Using induced pluripotent stem cell (iPSC)-derived presomitic mesoderm (PSM), we previously succeeded to quantify oscillatory activity of the segmentation clock, a molecular oscillator believed to control segmentation process (Matsuda, Yamanaka et al., Nature 2020; Matsuda et al., Science 2020). Interestingly, these in vitro models of the segmentation clock did not show any sign of segmentation or somitogenesis despite the presence of oscillatory activity of clock genes such as HES7. Extending on these earlier findings we then asked whether we could recapitulate not only the clock but also the actual process of segmentation and epithelial somite formation in vitro. Utilizing again pluripotent stem cells as starting material we succeeded to establish a 3D in vitro model of human somitogenesis, which exhibited periodic formation of properly patterned epithelial somites in synchrony with the segmentation clock (Yamanaka, Hamidi et al., Nature 2023). Our selforganizing ‘axioloids’ reconstituted various morphological and molecular features of the emerging human embryonic axis and could be also used to study the pathogenesis of human congenital diseases of the spine. Currently, we are continuing to improve and utilize axioloids and other in vitro models of embryonic development to increase our still limited understanding of development and disease in human and other primates.
Biography: Dr. Alev is a principal investigator and tenured faculty at the Institute for the Advanced Study of Human Biology (ASHBi) in Kyoto University. Together with his team at ASHBi he studies various aspects of early human embryonic development using stem cell-based model systems. Establishing and utilizing a variety of human and other species in vitro model systems of embryonic development & organogenesis, his lab at ASHBi aims to increase our still limited understanding of our own species’ biology, including human development, disease and evolution.
Dr. Alev did his postdoctoral work at the RIKEN Center for Developmental Biology (RIKEN CDB) in the laboratory of Dr. Guojun Sheng on gastrulation and post-gastrulation development focusing on mesoderm induction and patterning. During his subsequent tenure at the Center for iPS Cell Research and Application (CiRA) he used his insights into embryonic development to establish & study pluripotent stem cell-based models of human mesoderm development & differentiation, including in vitro models of the segmentation clock and diseases of the spine. In his lab at ASHBi Dr. Alev continues to work on ethically non-controversial in vitro models which can reconstitute defined aspects of human development in a dish.

Speaker: Dr. Vijay G. Sankaran, Jan Ellen Paradise, MD Associate Professor of Pediatrics, Boston Children's Hospital & Harvard Medical School
Topic: Genetic Influences on Human Hematopoiesis
Time: 9:30 AM (HKT)
Zoom: Here
CTSCB Stem Cell Seminar Series
Date: June 11, 2024
Abstract: In this presentation, I will discuss work from our laboratory exploring how naturally occurring genetic variation in humans can alter the process of blood and immune cell production, or hematopoiesis, in health and disease. I will illustrate how integrating human genetic studies with diverse functional methods offers profound mechanistic insights, enhancing our understanding of hematopoiesis. Additionally, I will highlight how our advances in creating more sophisticated tools for monitoring clonal dynamics significantly deepens our insights into this critical biological process.
Biography: Vijay G. Sankaran, MD, PhD is the Jan Ellen Paradise, MD Associate Professor of Pediatrics at Harvard Medical School, an Attending Physician in the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, and an Associate Member of the Broad Institute. Dr. Sankaran's lab seeks to understand the influence of human genetic variation on blood and immune cell production in health and disease. Their work has resulted in a number of therapies for blood diseases, including work that led to the development of Casgevy for sickle cell disease and beta-thalassemia. Dr. Sankaran has received a number of awards for his work including the 2018 Gale and Ira Drukier Prize in Children’s Health Research, the 2019 Seldin-Smith Award for Pioneering Research from the American Society of Clinical Investigation, and the 2022 E. Mead Johnson Award from the Society for Pediatric Research. He is an elected member of the Association of American Physicians and the American Society of Clinical Investigation.

Speaker: Dr. Joe Z Zhang, Principal Investigator, Institute of Metabolic and Cardiovascular Research, Shenzhen Bay Laboratory
Topic: Applications of hiPSCs in Cardiovascular Precision Medicine
Time: 11:30 AM – 12:45 PM (HKT)
Venue: INNO², 2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: In-person at HK Science Park & Online via Zoom
CTSCB Invited Seminar
Date: May 14, 2024
Abstract: The advent of human induced pluripotent stem cells (hiPSCs) has benefited many fields, from regenerative medicine to disease modeling, with an especially profound effect on cardiac research. hiPSCs offer a great opportunity to delineate human cardiac lineages, investigate inherited cardiovascular diseases, and assess the safety and efficacy of drugs. In this talk, I will provide several examples of application of hiPSCs in cardiovascular research, with a particular focus on modeling cardiac fibrosis using patient-specific hiPSCs. In the current work, we investigate the interaction between hiPSC-derived cardiomyocytes (hiPSC-CMs) and cardiac fibroblasts, leading to the trans-differentiation of cardiac fibroblasts into myofibroblasts which promote the process of cardiac fibrosis. This provides further evidence of feasibility of using hiPSCs to investigate the cross-talk among cells in cardiovascular precision medicine.
Biography: Dr. Zhang is a Principal Investigator (PI) at Shenzhen Bay Laboratory (SZBL), Shenzhen, China. He received his Ph.D. degree at the University of Otago, in 2015, followed by postdoctoral training at Stanford University from 2016-2020 and Instructor position at Stanford University from 2020-2021. Since 2021 September, he joined SZBL as a PI, and in the same year, he was awarded the Excellent Young Investigator Grant (overseas) from NSFC, China. His research focuses on Stem Cell and Cardiovascular Precision Medicine.
Based on previous contributions, Dr. Zhang have authored 29 articles, published in esteemed journals such as Cell Stem Cell, Circulation etc. Notably, 22 of these articles pertain to the applications of hiPSCs in cardiovascular precision medicine research. His major contributions to the scientific field include: 1. Generation of a dual-color lineage tracing hiPSC fluorescent reporter, enabling the simultaneous isolation of four distinct human cardiac lineages-an advancement that holds great potential for understanding cardiac development; 2. By employing patient-specific hiPSCs, he and his collaborators validated the pathogenicity of Variants of Uncertain Significance (VUS) in causing cardiovascular diseases. This pioneering work illustrates the utility of hiPSCs in assessing disease risk and aiding clinical diagnosis.


Speaker: Prof. Wolf Reik, Director, Altos Labs Cambridge Institute of Science
Topic: Single Cell Multi-Omics Landscape of Development and Ageing
Time: 4:30 PM (HKT)
CTSCB Stem Cell Seminar Series
Date: February 28, 2024
Abstract: Epigenetic information is relatively stable in somatic cells but is reprogrammed on a genome wide scale in germ cells and early embryos. Reprogramming is essential for imprinting, the return to totipotency and pluripotency, the erasure of epimutations, and for the control of transposons. Following reprogramming, epigenetic marking occurs prior to and during lineage commitment in the embryo. The epigenome changes in a potentially programmed fashion during the ageing process; this epigenetic ageing clock seems to be conserved in mammals.
Our work addresses the mechanisms and consequences of global epigenetic reprogramming in the early embryo, which is followed by epigenetic programming during lineage commitment. Using single cell multi-omics techniques, we are charting the epigenetic and transcriptional dynamics and heterogeneity during the exit from pluripotency and initial cell fate decisions leading up to gastrulation and organogenesis. We discovered priming of enhancers prior to lineage decisions as well as acute epigenetic remodeling of enhancers at the time of lineage commitment. We are also interested in the potentially programmed degradation of epigenetic information during the ageing process, how this might be coordinated across tissues and individual cells, and how this process potentially could be reversed to rejuvenate cells, tissues, and organisms.
Biography: Wolf Reik obtained his MD from the University of Hamburg. He did his thesis work with Rudolf Jaenisch, and postdoctoral work with Azim Surani in Cambridge. He became a Fellow of the Lister Institute of Preventive Medicine at Cambridge and subsequently the Head of the Epigenetics Programme at the Babraham Institute in Cambridge and was until recently its Director. He is honorary Professor of Epigenetics at the University of Cambridge, Associate Faculty at the Sanger Institute, where he is a founding member of the Centre for Single Cell Genomics, Affiliate Faculty at the Stem Cell Institute, and a member of the Centre for Trophoblast Research. He is a member of EMBO, Fellow of the Academy of Medical Sciences, Fellow of the Royal Society, and a member of the Academia Europaea. He is since March 2022 the founding Director of the Altos Labs Cambridge Institute of Science.
The research interests of his lab are in epigenetics, particularly in epigenetic reprogramming during mammalian development and its role in stem cell biology, inheritance, and ageing. Their current work addresses the mechanisms of genome-wide demethylation in the mammalian germ line, links between reprogramming, zygotic genome activation and pluripotency, epigenetic regulation of cell fate decisions in early development, and the role of epigenetic mechanisms in ageing and rejuvenation. The lab also develops new epigenomics technologies especially in single cells.

Speaker: Dr. Zheng Hu, Principal Investigator, Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences (CAS)
Topic: Mapping Cell Fate and Tumor Evolution with Single-Cell Lineage Tracing
Time: 11:30 AM – 12:45 PM (HKT)
Venue: INNO², 2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: In-person at HK Science Park & Online via Zoom
CTSCB Invited Seminar
Date: February 20, 2024
Abstract: High-resolution lineage tracing technology provides unprecedented opportunity for studying cell-fate decision and cancer evolution. The recent combination of singel-cell omics and lineage tracing enables systematic mapping of cell fate trajectory and discovery of fate-determining mechanisms. In the first part of my talk, I will introduce a computational method we recently developed (named PhyloVelo) for inferring cell-state transitions by integtracing single-cell RNA-seq and lineage tracing infromation. We demonstrated the high accuracy and robustness of PhyloVelo in inferring complex cell trajectories while outperforming RNA velocity, through applying it to different lineage-traced scRNA-seq datasets. In the second part, I will talk about the application of high-resoltion lineage tracing to decipher the origin and evolution of early preneoplastic lesions in mouse model of colorectal cancers. Our systematic lineage mapping reveals polyclonal-to-monoclonal transition during preneoplastic evolution.
Biography: Dr. Zheng Hu is a Principal Investigator at Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences (CAS). He is also the Director of Center for Synthetic Biology and Evolution at SIAT. Dr. Hu received a B.S. in Biomedical Engineering from Huazhong University of Science and Technology in 2010. He received his Ph.D in Evolutionary Genetics from Beijing Institute of Genomics CAS in 2015. From 2015 to 2020, he was a postdoctoral fellow in Dr Chrsitina Curtis’s lab at Stanford University School of Medicine. Dr Hu’s research interests span from cancer genomics, cancer evolution to computational modeling. His reseach of measuring the cancer evolutionary dynamics has yielded novel insights into cancer formation and metastasis, facilitating biomarker discovery for risk prediction and treatment decision making.

Speaker: Prof. Chengqi Lin, Professor, School of Life Science and Technology, Southeast University
Topic: Transcriptional and Epigenetic Control in Early Development
Time: 2:30 PM – 3:45 PM (HKT)
Venue: INNO², 2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: In-person at HK Science Park & Online via Zoom
CTSCB Invited Seminar
Date: January 23, 2024
Abstract: Promoter-proximal pausing of RNA Polymerase II (Pol II) is a critical and prevalence step in controlling the proper expression of key developmental genes throughout development, and its misregulation can lead to developmental defects and cancer pathogenesis. RNA Pol II mediated transcriptional elongation checkpoint (TEC) is regulated by P-TEFb-containing complexes through releasing paused Pol II into productive elongation. We have identified the most active form of P-TEFb within the Super Elongation complex (SEC), which is required for the rapid transcriptional induction of genes. Our recent studies indicated that the pausing and elongation regulator SPT5 undergoes phase transition during transcriptional pause release. During early elongation, the super elongation complex (SEC) induces SPT5 transition into the elongation droplets, which is essential for rapid gene induction. We will also discuss the roles of SEC in early development.

Speaker: Dr. Kyle G. Daniels, Assistant Professor of Genetics, Stanford University
Topic: Decoding the Language of Signaling Domains to Control Cell Function
Time: 10 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: January 16, 2024
Abstract: Cell therapies are powerful technologies in which human cells are reprogrammed for therapeutic applications such as killing cancer cells or replacing defective cells. The technologies underlying cell therapies are increasingly complexity, making rational engineering of cell therapies more difficult. Creating the next generation of cell therapies will require improved experimental approaches and predictive models. Artificial intelligence (AI) and machine learning (ML) methods have revolutionized several fields in biology including genome annotation, protein structure prediction, and enzyme design. Combining experimental library screens and AI to build create predictive models, design rules, and improved designs could accelerate the development of cell therapies. Chimeric antigen receptor (CAR) costimulatory domains derived from native immune receptors steer the phenotypic output of therapeutic T cells. We constructed a library of CARs containing ~2,300 synthetic costimulatory domains, built from combinations of 13 signaling motifs. These CARs promoted diverse cell fates, which were sensitive to motif combinations and configurations. Neural networks trained to decode the combinatorial grammar of CAR signaling motifs allowed extraction of key design rules. For example, non-native combinations of motifs which bind tumor necrosis factor receptor-associated factors (TRAFs) and phospholipase C gamma 1 (PLCg1) enhanced cytotoxicity and stemness associated with effective tumor killing. Thus, libraries built from minimal building blocks of signaling, combined with machine learning, can efficiently guide engineering of receptors with desired phenotypes. Similar approaches can be applied to design synthetic cytokine receptors that enhance CAR T cell function.

Speaker: Dr. Gui-Wei He, Junior Principal Investigator, Institute of Infectious Diseases, Shenzhen Bay Laboratory
Topic: Human Intestinal Organoids for Disease Modeling
Time: 3:45 PM – 5 PM (HKT)
Venue: INNO², 2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: In-person at HK Science Park & Online via Zoom
CTSCB Invited Seminar
Date: December 12, 2023
Abstract: Organoid technology is revolutionizing our way of modeling human diseases and developing new therapies. My laboratory aims to advance the application of human adult stem cell-derived organoids in diseases research. We combine bioengineering, CRISPR gene editing and single-cell RNA sequencing to develop novel human organoids that with increased physiological and pathological relevance. Meanwhile, we use human organoids as a model system to dissect mechanisms of human diseases, particularly infectious disease and cancer of the digestive system.
Biography: Dr. Gui-Wei He received his PhD degree with summa cum laude from the University of Erlangen-Nuremberg in Germany in 2018. Then Dr. He joined Professor Hans Clevers laboratory at the Hubrecht Institute, where he developed a next-generation human intestinal organoid model and used this new model system to study human intestinal development and diseases. Dr. He's studies were published in high-profile journals, including Cell Stem Cell, Gut and Journal of Experimental Medicine.
Speaker: Prof. Thomas Braun, Director, Max-Planck-Institute for Heart and Lung Research, Bad Nauheim, Germany
Topic: Plasticity of Cardiomyocytes During Development and Heart Regeneration
Time: 3:30 PM – 4:45 PM (HKT)
Venue: INNO², 2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: In-person at HK Science Park & Online via Zoom
CTSCB Invited Seminar
Date: November 28, 2023
Abstract: Ischemic cardiomyopathy is the single largest cause of death in countries of all income groups. Remarkable progress in the treatment of acute myocardial infarctions has improved the situation but the loss of myocardial tissue eventually leads to heart failure, showing an alarming increase in recent years. Unfortunately, the heart is a notorious non-regenerative organ, composed of terminally differentiated cardiac muscle cells, which has a very low renewal rate during adulthood, incapable of efficient organ regeneration after massive insults. This view is gradually changing as heart repair mechanisms have been discovered that are initiated in adult mammals following injuries. Clinically, the picture is less optimistic and, frustratingly, most clinical trials failed to yield novel treatments for this unmet need, illustrating the necessity to develop novel therapeutic approaches.
Mammalian cardiomyocytes proliferate during embryogenic heart development but loose their proliferative potential shortly after birth concomitant with morphological maturation and a profound switch of the cellular metabolism. Morphological maturation fills adult mammalian cardiomyocytes with sarcomeres and mitochondria, resulting in a pseudo-crystalline structure, which prevents cytokinesis. Reversal of this process, essentially rewinding the developmental program, to achieve a more immature, fetal state allows division of cardiomyocytes is essential for replacement of lost contractile tissue and successful organ regeneration. We have pursued several different strategies to rewind the development program to stimulate heart regeneration. We discovered that microRNAs of the miR-1/133a family are instrumental for suppression of two crucial regulatory circuits controlling postnatal cardiomyocyte proliferation and dedifferentiation, the FGFR and OSMR pathways. Concomitant inactivation of both miR gene clusters in postnatal cardiomyocytes resulted in expression of cell cycle regulatory genes and cell-cycle re-entry of adult cardiomyocytes. We also found that heart-specific expression of OSKM (Oct4(O), Sox2(S), Klf4(K) and c-Myc(M)) converts cardiomyocytes to a fetal-like state, conferring regenerative capacity to adult hearts. Short-term OSKM expression before and during myocardial infarction ameliorated myocardial damage and improved cardiac function, demonstrating that temporally controlled dedifferentiation and reprogramming enables cell cycle reentry of cardiomyocytes and facilitates heart regeneration. Inactivation of the miR-1/133a gene family as well as short-term cardiomyocyte-specific expression of OSKM reprogrammed the metabolism of heart muscle cells from oxidative phosphorylation to more anaerobic energy-producing pathways. We therefore asked whether abrogation of fatty acid oxidation (FAO) in cardiomyocytes is sufficient to induce cardiomyocyte de-differentiation, proliferation and eventually heart regeneration. Our results indicate that this is indeed the case, demonstrating that inhibition of FAO specifically in adult hearts after ischemic injury promotes nearly full recovery of cardiac function, providing fascinating perspectives for future clinical applications.
Biography: Thomas Braun is director at the Max-Planck-Institute for Heart and Lung Reseach, Bad Nauheim, Germany and Professur of Medicine at the Justus-Liebig-University Giessen, Germany. He studied medicine and philosophy at the Universities of Göttingen and Hamburg, where he obtained his MD and MD PhD. After postdoctoral training in Hamburg and in Boston in the lab of Rudolf Jaenisch at the Whitehead Insitute, MIT he became group leader at the Technical University of Braunschweig in 1992 before he moved on to an associate professor position at the University of Würzburg in 1996. After that he was appointed full professor and chair of Physiological Chemistry at the University of Halle-Wittenberg. In 2004 he was recruited by the Max-Planck-Society as founding director of the newly established Max-Planck-Institute for Heart and Lung Research in Bad Nauheim. Since 2004 he is also Professor of Medicine at the University of Giessen, Germany. So far, he has published close to 400 papers in leading journals including Nature, Science, Nature Medicine, Cell, Cell Stem Cell, Circulation, Circ. Res. and others His primary research currently focuses on the mechanisms driving skeletal and cardiac muscle development, regeneration and remodeling. He serves on various committees and advisory boards in Germany and abroad. He is an elected member of the German National Academy of Science, Leopoldina and the Academy of Europe and is editorial board member of several journals. Furthermore, he is in the steering board of several national and international research consortia and one of the directors of the Cardiopulmonary Institute (Frankfurt, Bad Nauheim, Giessen).
Speaker: Prof. Thomas Graf, Senior Scientist, Center for Genomic Regulation, Barcelona, Spain
Topic: Transcription Factor Methylation Regulates Transdifferentiation Velocity and Lineage Directionality
Time: 4 PM (HKT)
CTSCB Stem Cell Seminar Series
Date: October 31, 2023
Abstract: The impact of post-translational modifications on development has garnered substantial attention in the context of histones; however, their significance for lineage-instructive transcription factors remains relatively unexplored. We will present work showing that a specific post-translational modification of C/EBPa is critical for lineage choice at important developmental bifurcations. In prior research we demonstrated that C/EBPa induces the efficient transdifferentiation of B lymphocytes into macrophages and described a mutation in arginine 35 hindering C/EBPa methylation by the methyltransferase Carm1. Remarkably, this mutation dramatically expedites the B cell to macrophage transdifferentiation process. Mechanistically, the mutant protein displays an increased affinity for its partner PU.1, resulting in an almost instant activation of macrophage enhancers and silencing of B cell enhancers. In a physiological context Carm1 also regulates the differentiation directionality of C/EBPa-expressing granulocyte/macrophage progenitors, as inhibition of the enzyme increases the formation of macrophages at the expense of granulocytes. In ongoing work, we discovered that C/EBPa is also expressed in pre-implantation embryos and overexpression experiments that it represents a novel regulator of trophectoderm specification. Intriguingly, here again Carm1 methylation regulates lineage directionality as the R35A mutant or the enzyme’s inhibition in cells induced by wild type C/EBPa favors the formation of trophectoderm. Collectively, our research suggests the existence of two distinct forms of C/EBPa regulated by Carm1 methylation: the unmethylated form drives differentiation towards macrophages and trophectoderm cells while the methylated form fosters alternative fates. The observed interplay between Carm1 and a lineage-instructive transcription factor in determining adult and embryonic cell fates extends Carm1's established role in targeting transcription factors implicated in muscle development and in human blood and lung cell malignancies.
Speaker: Prof. Bee Luan Khoo, Assistant Professor, Department of Biomedical Engineering, City University of Hong Kong
Topic: Microsystems for Personalised Medicine - Integrative Microfluidic-based Biosensors for Disease Detection
Time: 11:00 AM – 12:15 PM (HKT)
Venue: INNO², 2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: Hybrid
CTSCB Invited Seminar
Date: October 25, 2023
Abstract: Liquid biopsy, in conjunction with microfluidic diagnostics, signifies a pivotal advancement in personalized disease management. Within cancer, advancing malignancies release circulating tumor cells (CTCs), offering a diverse representation of cancer types within a patient. We have developed patient-derived models based on CTC-derived cell clusters within an integrated microfluidic platform that simulates the patient's microenvironment. Our methodology has been rigorously validated using 500+ samples from breast, lung, and head-neck cancer patients, with results obtained within two weeks. Establishing CTC-derived models presents challenges, curtailing their applicability in patient prognosis and treatment assessment. Our success rate in establishing disease models surpasses prevailing studies by a minimum twofold margin, reaching up to 80% for pre-treatment specimens. Clinical validation studies have revealed an inverse relationship between cluster-forming potential and 5-year overall patient survival. The cluster-forming phenotype correlates with treatment modality and cancer stage. The patient-derived CTC cluster models exhibit prognostic capability for novel treatments, such as neoadjuvant regimens incorporating paclitaxel/carboplatin and lapatinib. LIQBP enables real-time, personalized evaluation and multiparameter quantitative phenotyping by integrating disease models with our data-driven algorithm system. This approach provides insights facilitating the identification of specific patient cohort profiles and assessing treatment response in a label-free manner.
Biography: Dr. Bee Luan Khoo is currently an Assistant Professor of Biomedical Engineering at the City University of Hong Kong (CityU). Dr. Khoo got her Ph.D. degree from the National University of Singapore, working on tumor models for prognosis evaluation. As a senior postdoctoral associate in the Singapore-MIT Alliance for Research and Technology, she developed microfluidic-based tools for rare cell detection.
Now Dr. Khoo's research group is focusing on detecting, prognosis, and characterization of disease heterogeneity using multidisciplinary techniques, including the design and utilization of microfluidic devices for personalized cancer management and evaluation. Dr. Khoo has authored more than 63 articles in top journals such as Energy & Environmental Science, Chemical Engineering Journal, Small, Biosensors and Bioelectronics, Science Advances, Nature Protocols, etc., and held 11 patents pending or granted.Dr. Khoo was awarded the Young Investigator national grant award by the National Medical Research Council and the Young Investigator award to support projects in disease detection via the Interstellar Initiative, funded by the Japan Agency for Medical Research and Development (AMED) and the New York Academy of Sciences. Dr Khoo's team was award 2x Silver at the recent The International Exhibition of Inventions of Geneva (“IEIG”). She is also the recipient of Innovators under 35 (Asia; MIT Technology Review) for her work on microfluidic devices with direct clinical relevance.Apart from research, Dr Khoo and her team are also actively seeking opportunities to encourage young people to enter the field of biomedical engineering through the implementation of the Gifted and Talented Program schemes 2021-2023 organized by the Education Bureau, and received the CENG Outstanding Teaching Award for her efforts.
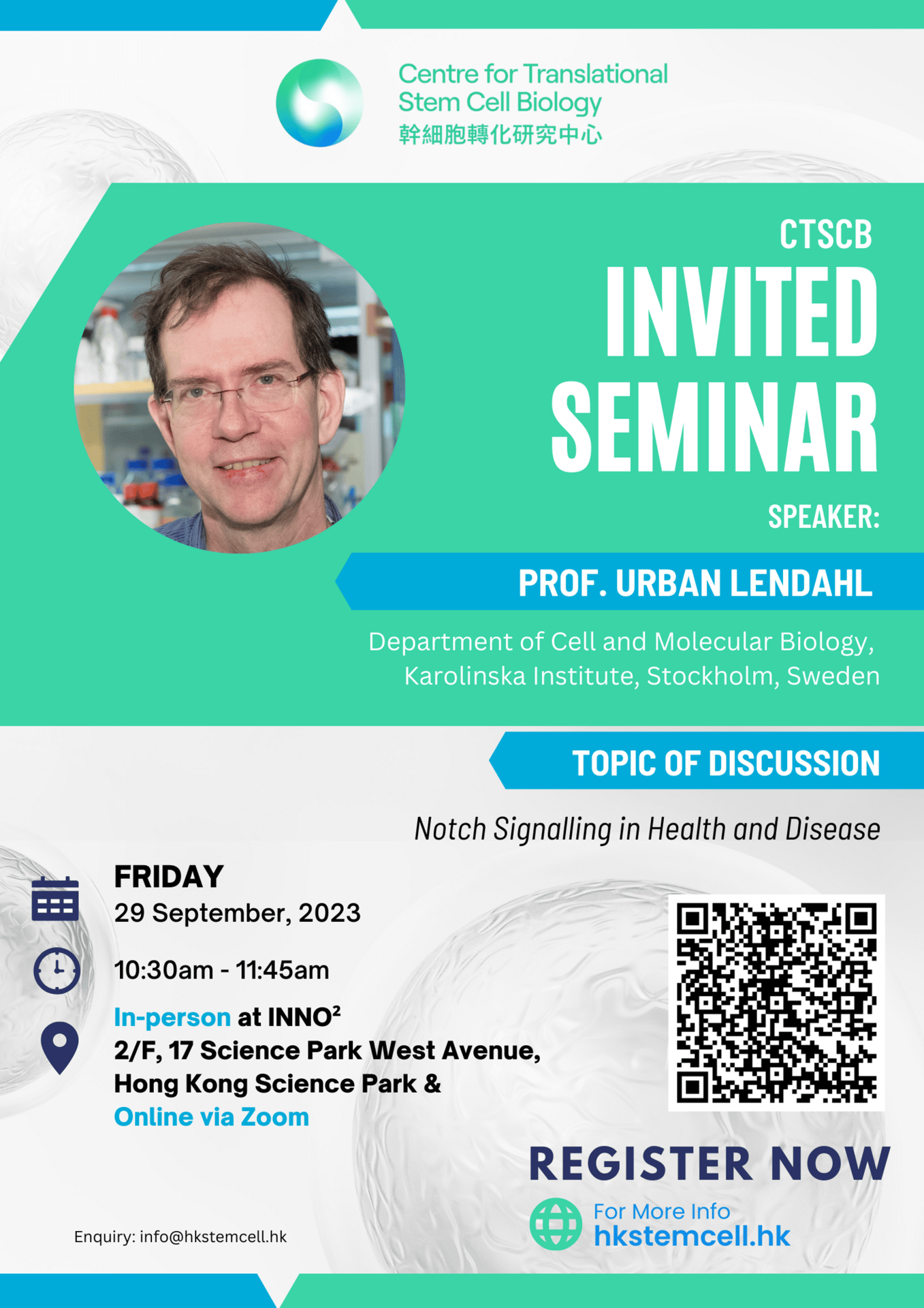
Speaker: Prof. Urban Lendahl, Department of Cell and Molecular Biology, Karolinska Institute, Stockholm, Sweden
Topic: Notch Signalling in Health and Disease
Time: 10:30 AM – 11:45 AM (HKT)
Venue: INNO², 2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: Hybrid
CTSCB Invited Seminar
Date: September 29, 2023
Abstract: In the lecture, I will discuss the role of Notch signalling in health and disease. Notch signalling is an evolutionarily conserved signaling mechanism that is pivotal for differentiation and homeostasis in most organs and tissues. Notch signalling has a simple architecture but generates considerable diversity in the signaling output in a cell context-dependent manner. I will provide examples of the roles of Notch in control of stem/progenitor cell differentiation but also discuss how Notch signaling is important for cellular homeostasis in different organs.
In the light of its importance in cellular differentiation, it is logical that dysregulated Notch signalling can lead to disease, and I will discuss data from two diseases caused by mutations in the Notch signalling pathway: Alagille syndrome, which is caused by mutations in the ligand JAGGED1 and the stroke and dementia syndrome CADASIL (Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy), a disease caused by mutations in the NOTCH3 gene. There is currently no therapy available for CADASIL patients, but I will describe recent preclinical testing of active immunization as a potential therapy strategy. Finally, I will discuss recent data on how an ailing vasculature caused by NOTCH3 mutations can lead to secondary problems for the brain’s immune regulation and the heart.
Biography: Dr. Urban Lendahl holds a PhD from Karolinska Institutet, Stockholm, Sweden (1987). During 1987-1989 he conducted postdoctoral work at Massachusetts Institute of Technology, with Professor Ron McKay as advisor. Lendahl is since 1997 Professor of Genetics at the Karolinska Institute.
Lendahl conducts research in the area of tumor biology, liver and vascular biology. The focus of his research is on the Notch signaling pathway and how dysregulated Notch signaling leads to disease, for example in liver and vascular diseases. Lendahl has also contributed to decoding the molecular architecture of the vascular system.
Lendahl has served as head of the Nobel Committee for Physiology and Medicine 2012 and as Secretary of the Nobel Assembly 2015. He has served as head of the review committee of the Human Frontiers Science Foundation, as a member of the Board of Directors of the International Society for Stem Cell Research (ISSCR), the Trustees Committee for the Koerber European Science Award as well as the Jury for the Heineken Prize. He holds a Distinguished Visiting Professorship at Hong Kong University and was awarded the Litchfield Lectureship with Title at Oxford University in 2018. Since 2022 Lendahl is the Secretary for the Sjoberg Prize in cancer research at the Royal Swedish Academy of Sciences.

Abstract: Induced Pluripotent Stem Cells (iPSCs) derived from somatic cells represent an invaluable cell source for therapeutic regenerative medicine. However, derivation of bona-fide iPSC is limited by low efficiency of the process and often heterogeneously reprogrammed cell populations. We have used multi-modal single-cell platform to analyze intermediate reprogramming cells to decipher the underlying mechanism governing epigenetic changes during the process. Unlike PSCs, Expanded Pluripotent Stem Cells (EPSC) manifest self-organizing ability in forming blastocyst-like structures upon induction. However, the intrinsic regulators orchestrating such blastoid forming potential remain unknown. We identified a nuclear receptor (NR) protein, which abrogation drastically reduces blastoid formation. Single chemical agonist for NR activation is sufficient in rewiring conventional Embryonic Stem Cells (ESC) towards a distinct pluripotency state capable of forming blastoids and contributing to embryonic and extraembryonic lineage in vivo. Mechanistically, we uncover a novel dual regulatory role of NR by interacting with corepressor or coactivator complex to modulate specific transposable elements cis-regulatory functions on target genes, to confer expanded pluripotency.
Biography: LOH Yuin-Han Jonathan is the Deputy Executive Director and Research Director at the A*STAR Institute of Molecular and Cell Biology (IMCB), where he also serves as the Director for the Cell Biology and Therapies Research Division. Concurrently, he is a Professor (Adjunct) at the National University Singapore (NUS) Yong Loo Lin School of Medicine and a Faculty member of the NUS Graduate School of Integrative Sciences and Engineering. He did his Ph.D. research in at the A*STAR Genome Institute of Singapore where he decoded the genetic and epigenetic regulation in embryonic stem cells (2006 Nature Genetics). He then moved to Boston Children Hospital at Harvard Medical School for postdoctoral training. There, he pioneered the use of reprogramming technologies to model hematopoietic diseases (2010 Nature; 2010 Cell Stem Cell). He joined IMCB as an A*STAR Investigator in 2011. His current research focuses on dissecting the mechanisms regulating cell fate changes, including how 1) epigenetic factors work with endogenous retro-element to coordinate gene expression programme (2015 Cell), 2) transcription factors direct transdifferentiation and somatic cell reprogramming (2016 Nat Commun.; 2020 Science Adv.), and 3) epitranscriptome regulation of cell states (2023 Molecular Cell). His research work has earned him several prestigious national and international accolades including the MIT TR35 Asia Pacific Award, Singapore Young Scientist Award. World Technology Network Fellowship, Stem Cell Society Singapore Outstanding Investigator Award, Entrepreneurship World Cup.
Date: September 7, 2023
CTSCB-HKU SBMS Joint Seminar
Speaker: Dr. Jonathan Yuin-Han Loh, Deputy Executive Director and Research Director, Cell Fate Engineering and Therapeutic Laboratory, A*STAR Institute of Molecular and Cell Biology (IMCB), Singapore
Topic: Stem Cell-Fate Engineering and Expanded Pluripotent State
Time: 2:30 PM – 4 PM (HKT) (HKT)
Venue: INNO², 2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: Hybrid
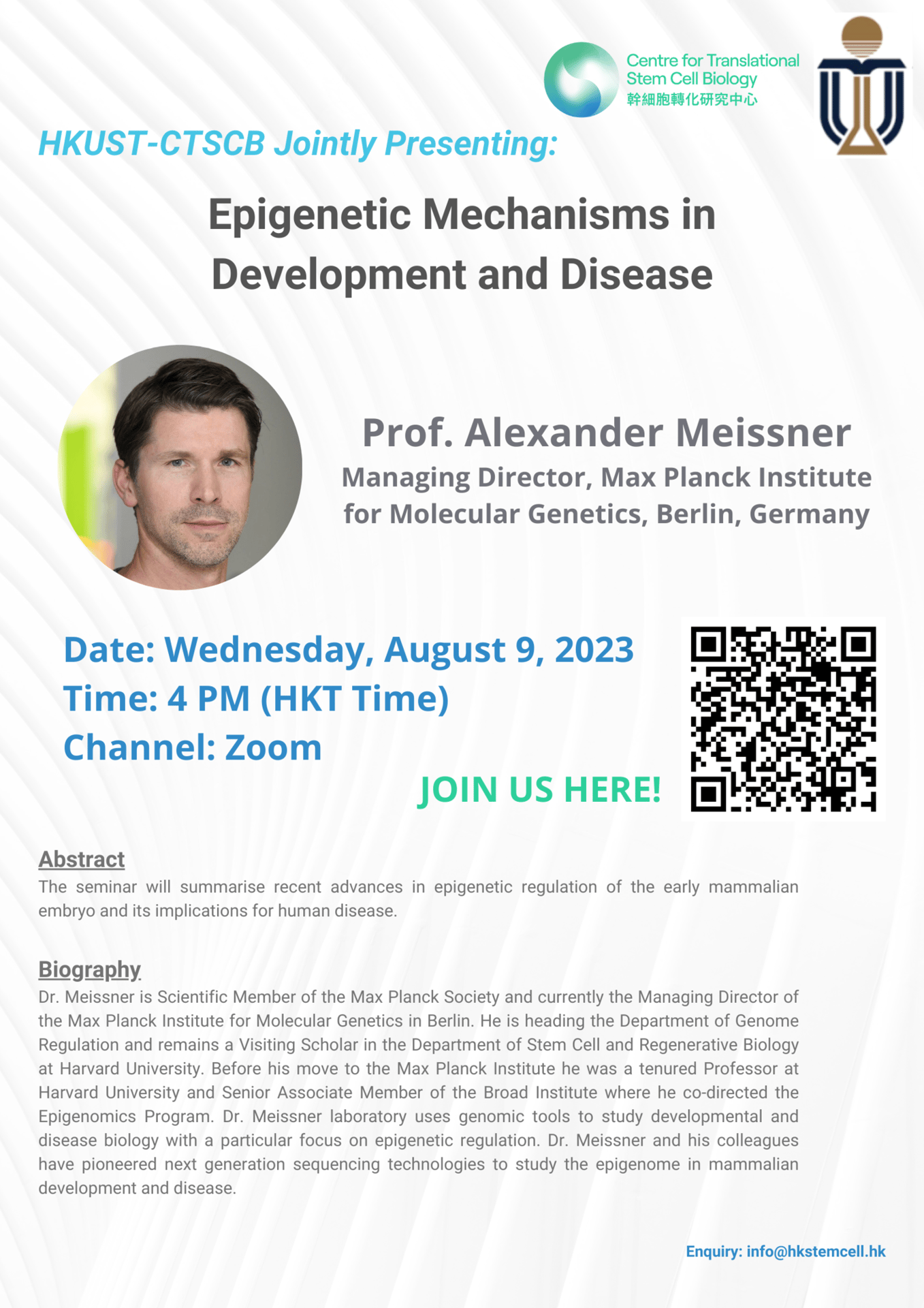
Date: August 9, 2023
CTSCB-HKUST Joint Seminar
Speaker: Prof. Alexander Meissner, Managing Director, Max Planck Institute for Molecular Genetics, Berlin, Germany
Topic: Epigenetic Mechanisms in Development and Disease
Time: 4 PM (HKT)
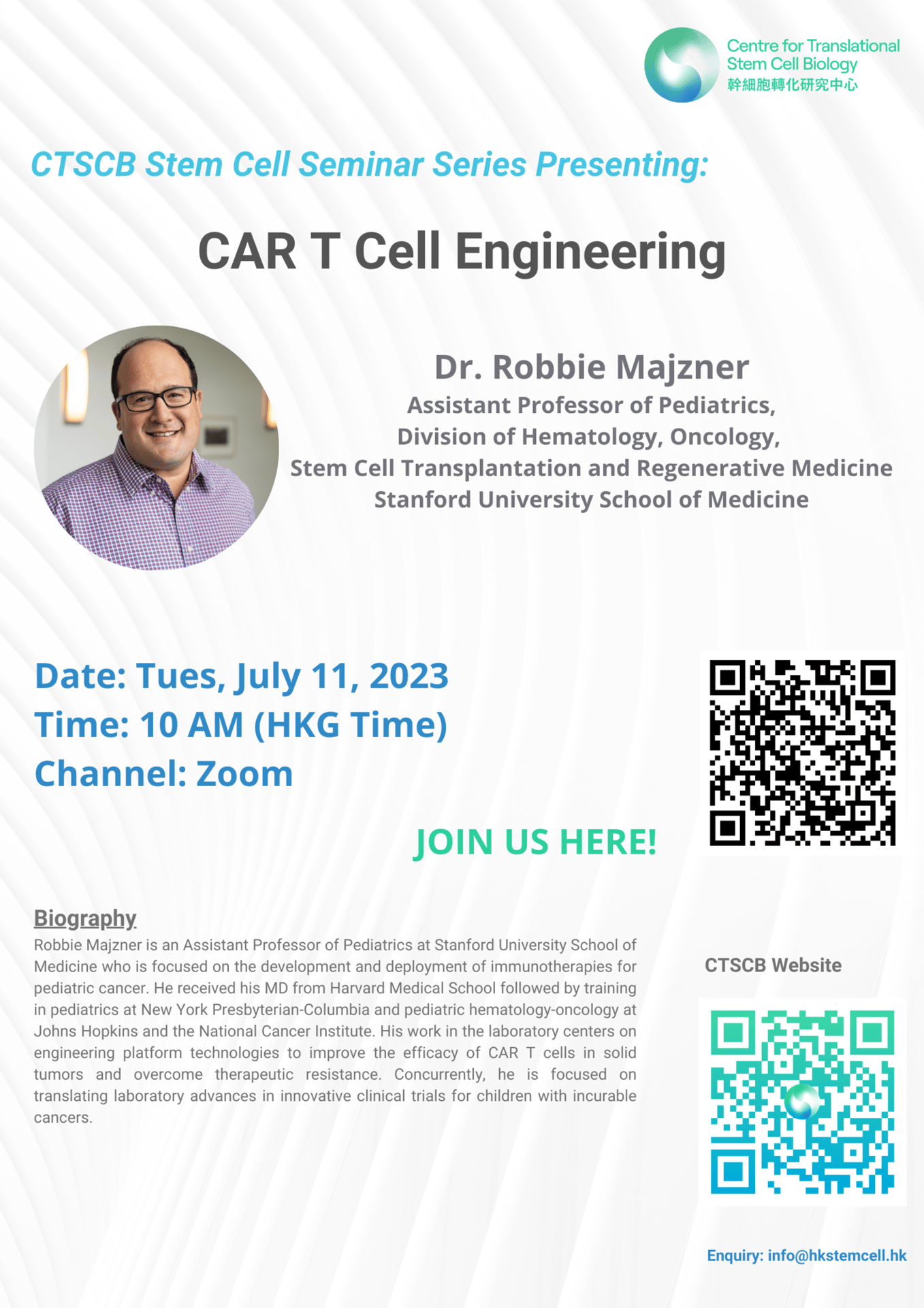
Date: July 11, 2023
CTSCB-HKUST Joint Seminar
Speaker: Dr. Robbie Majzner, Assistant Professor of Pediatrics, Division of Hematology, Oncology, Stem Cell Transplantation and Regenerative Medicine, Stanford University School of Medicine
Topic: CAR T Cell Engineering
Time: 10 AM (HKT)

Speaker: Prof. Alexander Hoffmann, Department of Microbiology, Immunology, and Molecular Genetics, Institute for Quantitative and Computational Biosciences, University of California, Los Angeles
Topic: How Specific are the Innate Immune Responses of Macrophages?
Time: 10:30 AM – 11:45 AM (HKT)
Venue: INNO², 2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: In-person at HK Science Park & Online via Zoom
CTSCB Invited Seminar
Date: June 20, 2023
Abstract: The immune system has two broad arms: innate immunity that is thought to provide general protection, and adaptive immunity that is capable of tailoring its responses with high specificity to foreign invaders. Yet, recent studies have revealed that macrophages, key immune sentinel cells that are the first to respond to pathogens and tissue injury, are capable of stimulus-specific responses. How stimulus-specific such innate immune responses remains unknown. I will present our recent studies to quantify the Specificity of Stimulus-Responses of macrophages and how they are affected by polarizing cytokines. As macrophage stimulus responses are highly dynamic and show cell-to-cell heterogeneity, our studies involve novel experimental measurement and computational analytical tools.
Biography: Alexander Hoffmann is the Thomas M Asher Professor of Microbiology and Immunology at UCLA, and the founding director of the Institute for Quantitative and Computational Biosciences (QCBio). Before joining UCLA in 2014, he was Professor of Biochemistry at UCSD; there he founded the San Diego Center for Systems Biology (SDCSB), the BioCircuits Institute (BCI), and directed the Graduate Program in Bioinformatics and Systems Biology.
In his PhD with Robert Roeder at Rockefeller University he cloned the TATA box binding protein (TBP), which specifies where RNA polymerase initiates transcription. In his postdoc with David Baltimore at MIT and Caltech, he discovered the dynamic control of NFκB and established the hypothesis that there is a Temporal Code in stimulus-responsive signaling systems. This has been a far-reaching concept that has informed studies on many signaling systems including MAPK, ERK, p53, interferon, and many others. His own laboratory characterized this Temporal Code and the mechanisms that enable it, and they reported the identification of “Signaling Codons”, dynamic features in NFκB activities that convey information to nuclear target genes.
His current research aims to develop a predictive understanding of immune responses, focusing on immune sentinel macrophages, on the generation of antibody responses by B-cells, and on the generation of new immune cells via hematopoiesis.

Speaker: Dr. Sidi Chen, Associate Professor, Department of Genetics, Systems Biology Institute, & Cancer Center, School of Medicine, Yale University
Topic: In Vivo Gene Editing and Immunotherapy
Time: 9 AM (HKT)
Date: June 14, 2023
Abstract: My vision is to understand the fundamental problems in the genetics and immunity of cancer and other human diseases, and to develop next-generation therapeutics. By intersecting two emerging fields, I envision a concept “genome engineering for, of, and as immunotherapy”. My group develop high-throughput genome engineering and screening technologies for the discovery of immunotherapy targets; perform genome engineering of next-generation therapeutic immune cells such as CAR-Ts; and harness genome engineering directly as novel immunotherapy modalities by re-directing the genetic manipulations towards immune modulation. My lab’s research uncovers novel insights in cancer and seeks to develop them into the next generation of therapeutics.
CTSCB Stem Cell Seminar Series

Speaker: Dr. Christoph Ziegenhain, Assistant Professor, Karolinska Institutet
Topic: Scalable Full-Length Single-Cell RNA-Seq to Decode Alternative mRNA Splicing
Time: 1:45 PM – 2:30 PM (HKT)
Venue: Studio, INNO², 2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: Hybrid
CTSCB-MGI Invited Seminar
Date: May 24, 2023
Abstract: Single-cell RNA-sequencing is widely used to map cell types and transcriptomic programs in health and disease. However, commonly used approaches can only sequence the ends of RNA molecules, limiting the scope of new discoveries. Here, I will discuss the development of an improved full-length scRNA-seq method that finally overcomes the long-standing compromise between cost, cellular throughput, and data quality. Excitingly, this technology opens up the possibility to map out cell-type usage and regulation of alternative splicing patterns.
Biography: Christoph completed his PhD studies at the University of Munich, Germany in 2017 where he was working on new single cell technology and evolutionary genomics. From 2018, he was a Postdoc with Prof. Rickard Sandberg at Karolinska Institute, developing high throughput full-length single cell methods and studying transcriptional kinetics. Now, he is starting his own group at Karolinska Institute with a focus on alternative splicing.

Speaker: Dr. Saar Gill, Associate Professor of Medicine, University of Pennsylvania
Topic: Targeting CD45 as a Pan-Hematologic Neoplasm Antigen
Time: 9 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: May 23, 2023
Abstract: CD45 is an attractive target for nearly all hematopoietic tumors but has been considered “undruggable” due to high levels of CD45 expression on stem cells and all cells of the adaptive and innate immune system. Gene deletion of CD45 is not possible because the tyrosine phosphatase domain is essential for function in stem cells and T cells. We have found that CRISPR base editing can install function preserving mutations on the extracellular domain of CD45. Therefore, CD45 CAR T cells avoid fratricide and CD45BE HSC and their progeny are not targeted by CD45 CARBE T cells. We provide preclinical data to support this approach as an effective cell therapy for AML; to date, AML has not been targeted with CAR T cells due to the shared antigenic targets on AML and the closely related HSC.
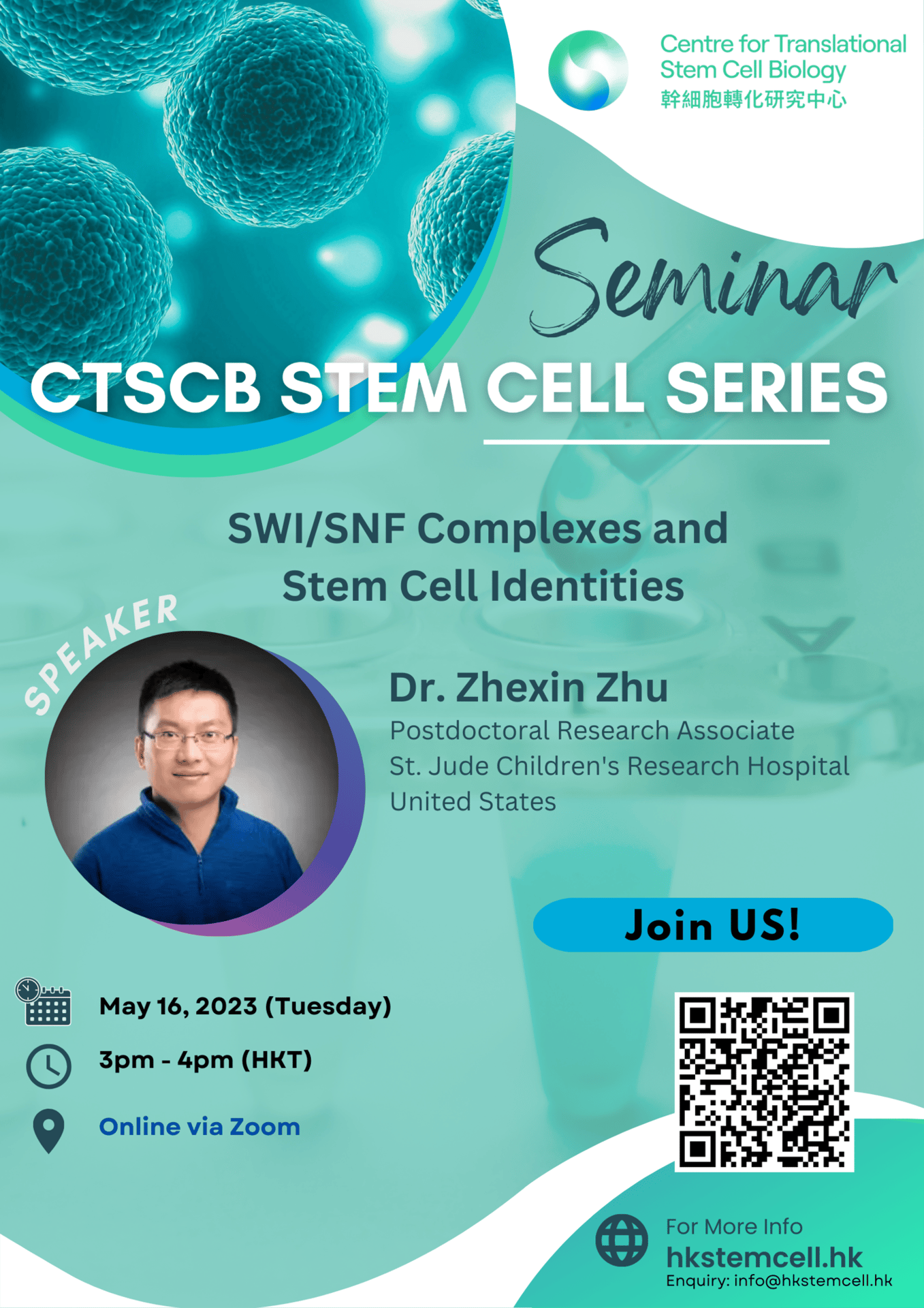
Speaker: Dr. Zhexin Zhu, Postdoctoral Researcg Associate, St. Jude Children's Research Hospital, United States
Topic: SWI/SNF Complexes and Stem Cell Identities
Time: 3 PM (HKT)
CTSCB Stem Cell Seminar Series
Date: May 16, 2023
Abstract: For cells to initiate and sustain a differentiated state, it is necessary that a ‘memory’ of this state is transmitted through mitosis to the daughter cells. Mammalian switch/ sucrose non-fermentable (SWI/SNF) complexes (also known as Brg1/Brg-associated factors, or BAF) control cell identity by modulating chromatin architecture to regulate gene expression, but whether they participate in cell fate memory is unclear. Here we provide evidence that subunits of SWI/SNF act as mitotic bookmarks to safeguard cell identity during cell division. The SWI/SNF core subunits SMARCE1 and SMARCB1 are displaced from enhancers but are bound to promoters during mitosis and we show that this binding is required for appropriate reactivation of bound genes after mitotic exit. Ablation of SMARCE1 during a single mitosis in mouse embryonic stem cells is sufficient to disrupt gene expression, impair the occupancy of several established bookmarks at a subset of their targets and cause aberrant neural differentiation. Thus, SWI/SNF subunit SMARCE1 has a mitotic bookmarking role and is essential for heritable epigenetic fidelity during transcriptional reprogramming.
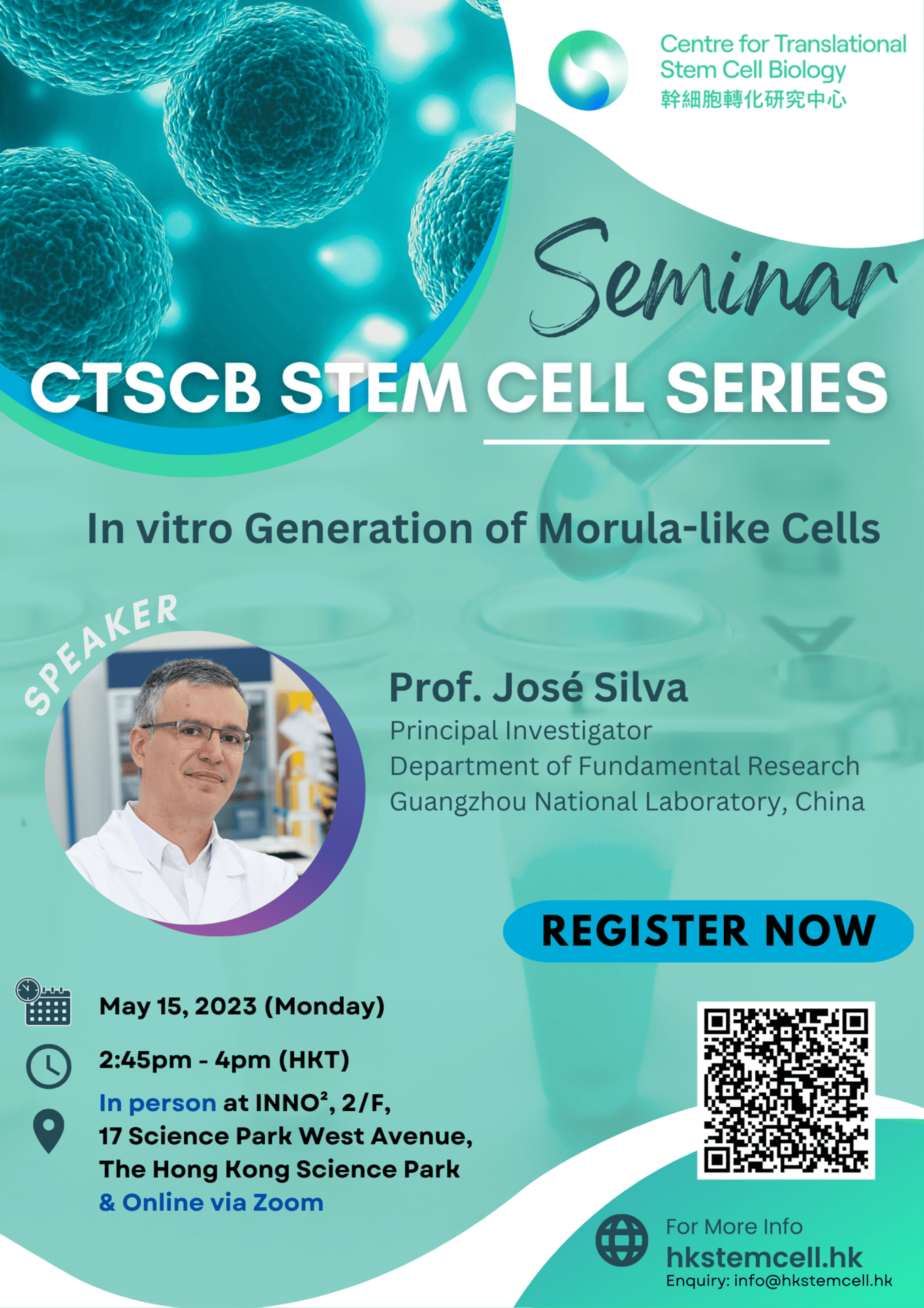
Speaker: Prof. José Silva, Principal Investigator, Department of Fundamental Research, Guangzhou National Laboratory, China
Topic: In vitro Generation of Morula-like Cells
Time: 2:45 PM – 4 PM (HKT)
Venue: INNO²,2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: Hybrid
CTSCB Stem Cell Seminar Series
Date: May 15, 2023
Abstract: Generating cells with molecular and functional properties of early embryo cells is an aim with fundamental biological and medical relevance. I will communicate on the in vitro generation of mouse transient totipotent morula-like cells (MLCs) via manipulation of signaling pathways. MLCs are molecularly distinct from embryonic stem cells and cluster instead with totipotent 8-16-cell stage embryo cells. Single MLCs can generate blastoids and efficiency increases to 80% when 8-10 MLCs are used. MLCs are also sufficient to efficiently generate gastrulating-like embryoids displaying all cell types of embryonic and extraembryonic origins. MLCs introduced into morulae develop into molecularly indistinguishable epiblast, primitive endoderm and trophectoderm fates. These findings are important for the harnessing of totipotency and for the creation of sophisticated and advanced in vitro embryoids from a single cell population.
Biography: José is a full-time Principal Investigator at the department of fundamental research at the Guangzhou National Laboratory. José is a former Principal Research Associate and Wellcome Trust Senior Research Fellow at the Wellcome-MRC Cambridge Stem Cell Institute at the University of Cambridge. He has served as a Principal Investigator at the University of Cambridge for 12 years and engaged in cell fate determination and stem cell related research. José is also known for his pioneering contributions to the research field of nuclear reprogramming. Jose’s current work is focused on the development of systems that can mimic early embryonic development in vitro. His goal with these systems is to be able to create in the laboratory desired cell types for applications in regenerative medicine. José has authored numerous publications as first and as corresponding author published in top tier scientific journals such as Cell, Nature and Cell Stem Cell, and these publications are highly cited. During his time at the University of Cambridge he won successively highly competitive and prestigious awards, including the Wellcome Trust Senior Research Fellowship Award. Recently, José was the recipient of the prestigious National Major Talent Award (China).
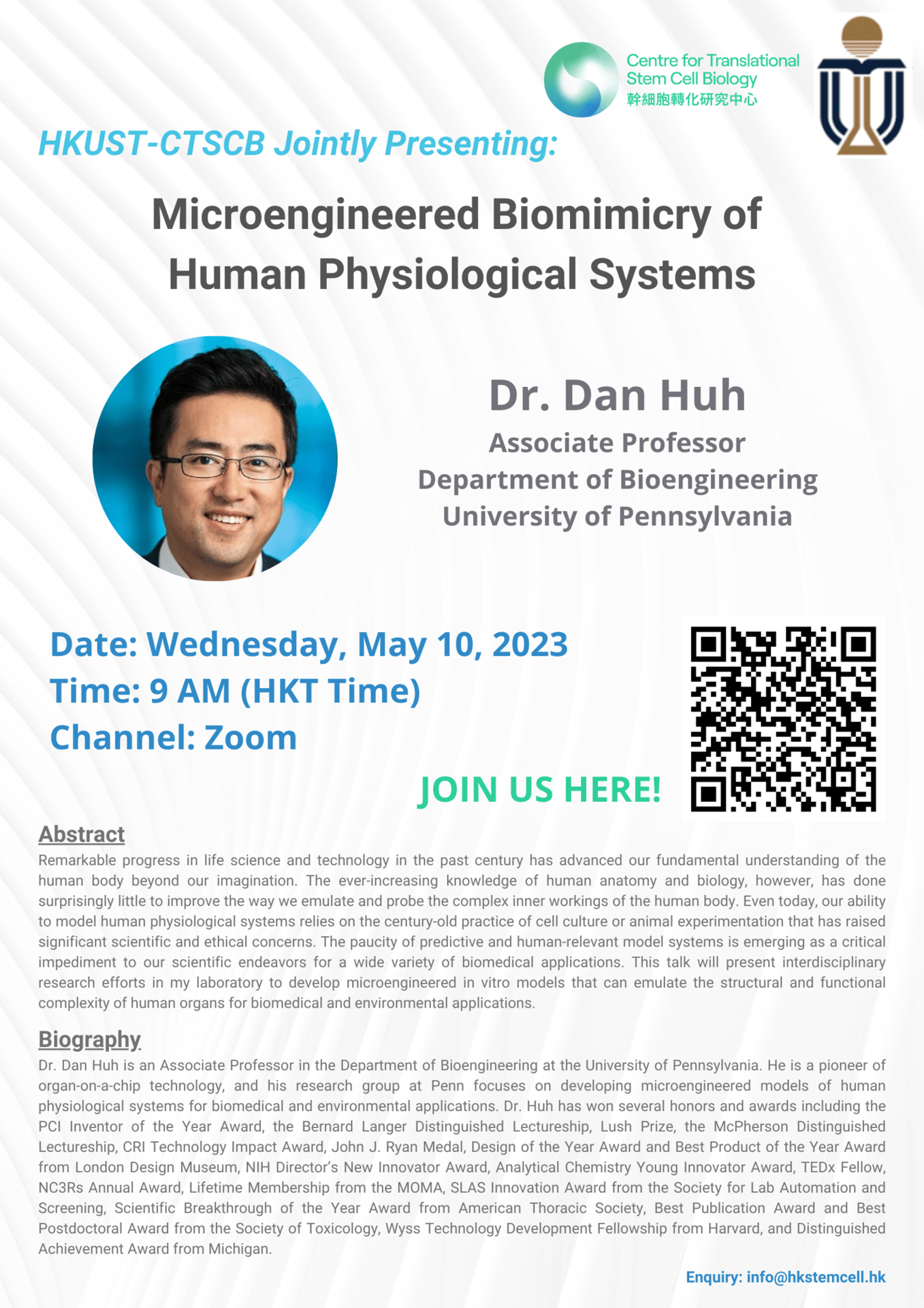
Speaker: Dr. Dan Huh, Associate Professor, Department of Bioengineering, University of Pennsylvania
Topic: Microengineered Biomimicry of Human Physiological Systems
Time: 9 AM (HKT)
CTSCB-HKUST Joint Seminar
Date: May 10, 2023
Abstract:Remarkable progress in life science and technology in the past century has advanced our fundamental understanding of the human body beyond our imagination. The ever-increasing knowledge of human anatomy and biology, however, has done surprisingly little to improve the way we emulate and probe the complex inner workings of the human body. Even today, our ability to model human physiological systems relies on the century-old practice of cell culture or animal experimentation that has raised significant scientific and ethical concerns. The paucity of predictive and human-relevant model systems is emerging as a critical im-pediment to our scientific endeavors for a wide variety of biomedical applications. This talk will present interdisciplinary research efforts in my laboratory to develop microengineered in vitro models that can emulate the structural and functional complexity of human organs for biomedical and environmental applications.
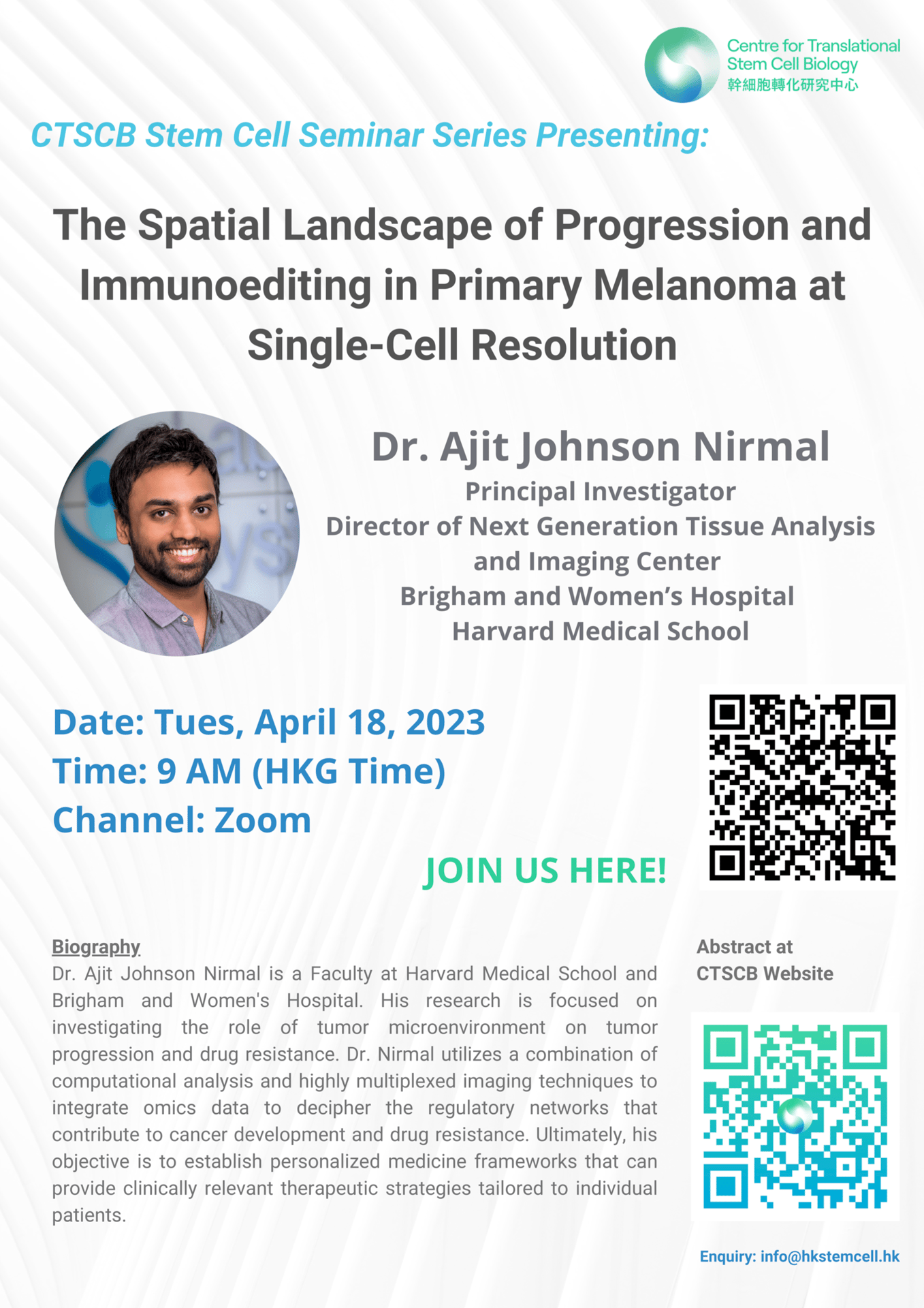
Speaker: Dr. Ajit Johnson Nirmal, Principal Investigator, Director of Next Generation Tissue Analysis and Imaging Center, Brigham and Women’s Hospital, Harvard Medical School
Topic: The Spatial Landscape of Progression and Immunoediting in Primary Melanoma at Single-Cell Resolution
Time: 9 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: April 18, 2023
Abstract: Cutaneous melanoma is a highly immunogenic malignancy that is surgically curable at early stages but life-threatening when metastatic. Here we integrate high-plex imaging, 3D high-resolution microscopy, and spatially resolved microregion transcriptomics to study immune evasion and immunoediting in primary melanoma. We find that recurrent cellular neighborhoods involving tumor, immune, and stromal cells change significantly along a progression axis involving precursor states, melanoma in situ, and invasive tumor. Hallmarks of immunosuppression are already detectable in precursor regions. When tumors become locally invasive, a consolidated and spatially restricted suppressive environment forms along the tumor–stromal boundary. This environment is established by cytokine gradients that promote expression of MHC-II and IDO1, and by PD1–PDL1-mediated cell contacts involving macrophages, dendritic cells, and T cells. A few millimeters away, cytotoxic T cells synapse with melanoma cells in fields of tumor regression. Thus, invasion and immunoediting can coexist within a few millimeters of each other in a single specimen.
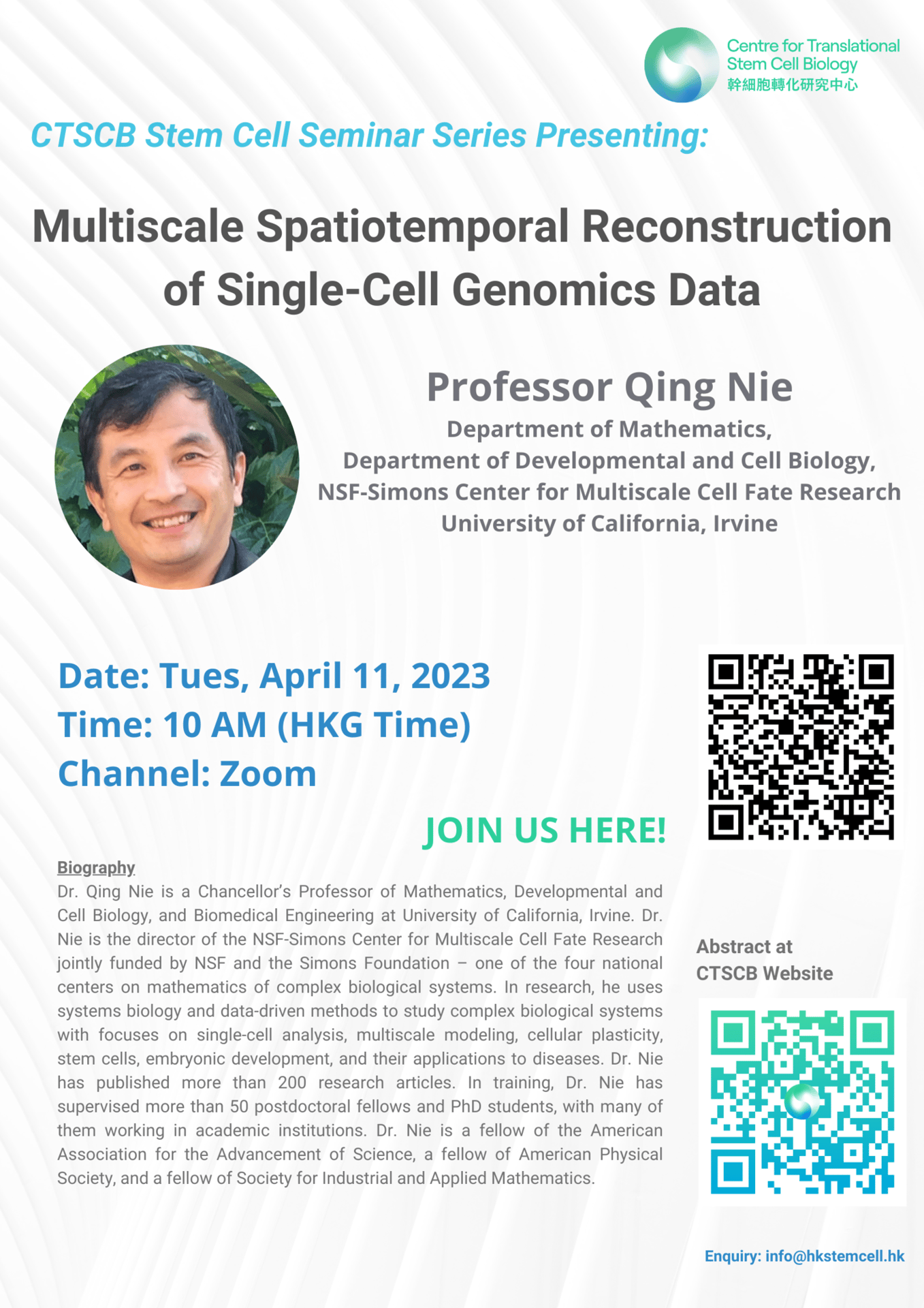
Speaker: Professor Qing Nie, Department of Mathematics, Department of Developmental and Cell Biology, NSF-Simons Center for Multiscale Cell Fate Research, University of California, Irvine
Topic: Multiscale Spatiotemporal Reconstruction of Single-Cell Genomics Data
Time: 10 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: April 11, 2023
Abstract: Cells make fate decisions in response to dynamic environments, and multicellular structures emerge from multiscale interplays among cells and genes in space and time. The recent single-cell genomics technology provides an unprecedented opportunity to profile cells. However, those measurements are taken as static snapshots of many individual cells that often lose spatiotemporal information. How to obtain temporal relationships among cells from such measurements? How to recover spatial interactions among cells, such as cell-cell communication? In this talk I will present our newly developed computational tools that dissect transition properties of cells and infer cell-cell communication based on nonspatial single-cell genomics data. In addition, I will present methods to derive multicellular spatiotemporal pattern from spatial transcriptomics datasets. Through applications of those methods to several complex systems in development, regeneration, and diseases, we show the discovery power of such methods and identify areas for further development for spatiotemporal reconstruction of single-cell genomics data.
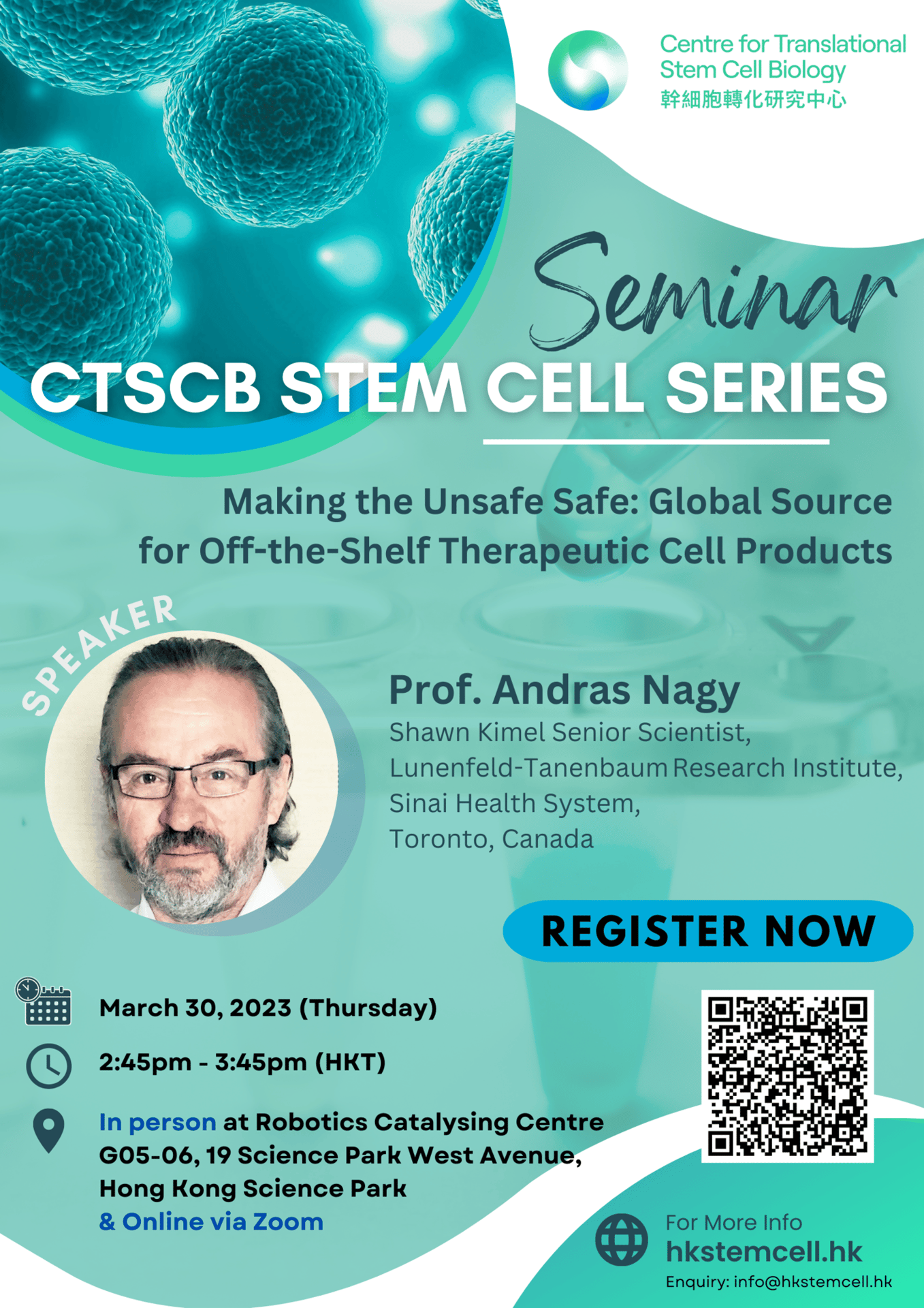
Speaker: Prof. Andras Nagy, Shawn Kimel Senior Scientist, Lunenfeld-Tanenbaum Research Institute, Sinai Health System, Toronto, Canada
Topic: Making the Unsafe Safe: Global Source for Off-the-Shelf Therapeutic Cell Products
Time: 2:45 PM – 3:45 PM (HKT)
Venue: Robotics Catalysing Centre, G05-06, 19 Science Park West Avenue, Hong Kong Science Park
Format: Hybrid
CTSCB Stem Cell Seminar Series
Date: March 30, 2023
Abstract: The use of ex-vivo cultured cells as a therapeutic means is currently moving into the clinic setting to treat devastating degenerative conditions. We have addressed one of the most urgent roadblocks for such cell therapies: the allograft tolerance without the need for immune suppression. We hypothesized that transgenic expression of eight local-acting, immune-modulatory transgenes is sufficient to protect cells against rejection and achieve induced Allogeneic Cell Tolerance (iACT) in fully immune-competent mice. Allografts survived long-term, in different MHC-mismatched recipients, and without the use of immunosuppressive drugs. The immune-modulatory genes have highly conserved functions, suggesting that the strategy would also work in the human system. iACT stem cells could serve as a source for off-the-shelf available cells to treat/cure medical conditions without the need for immune suppression of the patient.
However, it is critical to provide a steadfast level of safety in cell-based therapies involving evasion of immune rejection. The lack of immune surveillance on these cells increases the likelihood of developing cancer. To make the cells clinically relevant, we developed a solution for cell safety by building a highly reliable kill-switch into cells, allowing for the elimination of cells with cancerous potential or uncontrolled proliferation. This “FailSafeTM” cell system also enables the quantification of risk in the function of cell number needed for each specific therapeutical goal, which is critical to making informed decisions by the regulators, doctors, and patients to advance the modern medicine-transforming cell therapies.
The combination of the FailSafe™ cell and iACT genome editing allows for generating a “single” pluripotent cell line as a source of off-the-shelf available therapeutic cell products. These cells can be further edited to express biologics in order to attain other therapeutic effects explicitly targeting the underlying mechanism of the disease, for example, exerting anti-inflammatory or analgesic effects or even antibodies against infections. Some models will be presented for such a combination of gene and allogeneic cell therapy.
Biography: Dr. Nagy has made significant breakthroughs in developmental genetics, mouse and human pluripotent stem cell biology (both embryonic and reprogramming-induced), disease modelling and cell therapy approaches. His team created the first Canadian human embryonic stem cell lines in early 2000. In 2009, they developed the first method allowing the generation of iPS cell lines without any genetic change. Their approach allowed studying the reprogramming process at multiple OMICS levels, almost at daily resolution from differentiated cells to pluripotency. His current research has become even more translational by addressing and coming up with solutions for two significant hurdles of cell therapies: safety and allogeneic cell acceptance without the need for suppression of the immune system. Dr. Nagy’s research in cell-based therapy aims to advance medicine with a focus on treating incurable degenerative diseases, such as blindness, diabetes mellitus, arthritis, spinal cord injury, ageing, haemophilia, hypothyroidism, chronic pain, and multiple neurological disorders, including multiple sclerosis, depression, and bipolar disease.

Speaker: Professor Jianlong Wang, Professor (tenured), Department of Medicine, Columbia University Irving Medical Center (CUIMC), New York
Topic: Transcriptional and Translational Control of Stem Cell and Developmental Potency
Time: 9 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: March 28, 2023
Abstract: We employ genetic, genomic, and proteomic approaches combined with cellular and mouse models to dissect the regulatory layers encompassing transcriptional, translational, and epigenetic mechanisms underlying the distinct stem cell fates and early development. Our major efforts in studying mouse and human embryonic stem cell (hESC) maintenance have led to discovery of many transcription factors, noncoding miRNAs/lncRNAs, translation factors, and epigenetic regulators acting alone or in conjunction to maintain pluripotency. Totipotency, a remarkable cellular plasticity of a single cell in generating a complete organism with both embryonic and extraembryonic contribution, is essential for multicellularity and development. In mice, fully totipotent cells exist transiently in the zygote and cleavage-stage blastomeres, although totipotent 2-cell (2C)-like cells (2CLCs) can also arise sporadically or be induced genetically in cultured ESCs that are pluripotent and can only contribute to embryonic lineages. Recently, expanded potential stem cells or extended pluripotent stem cells, collectively known as EPSCs, with totipotency features were derived and stably cultured in vitro using two distinct sets of small molecule inhibitors. However, despite their functional similarities in embryonic and extraembryonic bipotential, 2CLCs and the two EPSC lines differ in their culture conditions, transcriptomes, and expression of core pluripotency factors NANOG/OCT4, suggestive of alternative totipotent states. Defining molecular pathways underlying these various totipotent cells will facilitate unraveling the complex regulatory mechanisms of totipotency and achieving the capture/stabilization of totipotent stem cells in culture. I will discuss our continuous efforts to understand the molecular control mechanisms of both pluripotency and totipotency.
Abstract: Exogenous expression of Oct4, Sox2, KLf4 and cMyc, convert somatic cells into iPSCs. This has not only made the generation of patient-derived pluripotent stem cells for regenerative medicine possible but also demonstrated the possibility to faithfully manipulate cellular identify by transcription factors (TFs) and supply desired cell types. Since the generation of iPSCs, many different cell types have been generated by cell type-specific master TFs. However, in general, TF-mediated cellular reprogramming is inefficient and often fails to generate fully functional cells. Even in the generation of iPSCs, only about 2-3% of the starting somatic cells can give rise to iPSCs at most. In order to understand the principles of TF-mediated reprogramming, we have recently performed CRISPR/Cas9-mediated genome-wide knockout screening during iPSC generation and then identified 16 roadblock genes that hinder reprogramming, as well as 16 genes essential for iPSC generation but not for ES cell self-renewal or MEF proliferation. I will present how those genes inhibit or facilitate iPSC generation as a model of TF-mediated cell conversions.
Biography: Keisuke Kaji is currently a professor and an MRC Senior Non-Clinical Fellow in the Centre for Regenerative Medicine, Institute for Regeneration and Repair, University of Edinburgh. He obtained his MSc and PhD in the Tokyo Institute of Technology, Japan, in the laboratory of Prof Akira Kudo. In the Kudo lab, he generated CD9 knockout mice and revealed that CD9 on the egg surface is critical for sperm-egg fusion (Nature Genetics, 2000, Dev Biol, 2003). In 2003, he moved to Edinburgh as a postdoc with JSPS fellowship. During his postdoc, he studied the roles of the NuRD co-repressor complex in pluripotent cells in the Hendrich lab (Nature Cell Biol, 2006, Development, 2007). In January 2008, he started his own group and succeeded in making a non-viral single vector reprogramming system using the piggyBac transposon (Nature, 2009). Since then, his group identified cell surface markers to track the reprogramming process (Nature, 2013), and revealed that Smad3 can enhance multiple transcription factor-mediated cell conversions (Cell Stem Cell, 2017). His lab has recently performed CRISPR genome-wide knockout screening during reprogramming and identified a SINE binding protein ZFP266 impeded reprogramming factor-mediated chromatin opening (Nature Communications, 2022). 4-5 years ago, his group has also started working on reprogramming mature hepatocytes into progenitors with efficient re-differentiation capacities, aiming to achieve an unlimited supply of fully functional hepatocytes.
Abstract: The devastating pandemic viruses have led to a global public health crisis and heavy economic losses. The use of animal-originated cells, the low efficiency of cell proliferation, and the lack of sensitive readouts are the bottlenecks in the vaccine industry and antiviral drug development. It is urgently needed to develop a new type of human cell model to fit industrial manufacturing.
We have established a stem cell-based system where human Expanded Potential Stem Cells (hEPSCs) were used to generate bona fide trophoblast stem cells (hTSCs) and trophoblast subtypes syncytiotrophoblasts (STBs) and extravillus trophoblasts (EVTs). We discovered that early STBs (eSTBs) express high levels of SARS-CoV-2 host factor angiotensin-converting enzyme 2 (ACE2) and are productively infected by SARS-CoV-2 and its Delta and Omicron variants to produce virions. Antiviral drugs suppress SARS-CoV-2 replication and effectively mitigated the virus infection in eSTBs. Remarkably, the infected eSTBs are a few orders of magnitude (1000 times) more sensitive to the antiviral drugs. Stem cell-derived human trophoblasts such as eSTBs can potentially provide unlimited amounts of normal and genome-edited cells and facilitate coronavirus research and antiviral discovery.
The proposed products include the pre-GMP grade cell products derived from human EPSC for studying various of viruses, including but not limited to SARS-CoV-2, MERS-CoV, their variants, and the influenza (Flu) virus. Antiviral drug candidates including the FDA approved drugs and natural products, will also be provided by using the stem cell-based platform.
Biography: Dr. Degong Ruan obtained his Ph.D. qualification in Development Biology at the Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences. Starting his academic career development at The University of Hong Kong, Dr. Ruan engaged in the project of trophoblast stem cells from human EPSC differentiation in the lab of Professor Pentao Liu. During his time at HKU, working along with Professor William Yeung's team from the HKU Department of O&G, Dr. Ruan established stem cell (iPSC) lines from human, pig, and mouse embryos. After a few years of academic training at HKU, Dr. Ruan decided to join the innovative program developed by Prof. Pentao Liu, with the support from Innovation and Technology Commission. He successfully patented the blastoid technology from the program with a jounral published in Cell Reports Medicine in late 2022.

Speaker: Dr. Degong Ruan. Single Cell Sequencing Platform PIC, Centre for Translational Stem Cell Biology
Topic: A Novel Stem Cell-Based Platform for Antiviral Discovery, Vaccine and Healthy Natural Products
Joint Seminar by Centre for Translational Stem Cell Biology & Institute for Regeneration and Repair
Date: March 22, 2023
Time: 10:30 AM – 12:30 AM (HKT)
Venue: INNO², 2/F, 17 Science Park West Avenue, Hong Kong Science Park
Format: In-person
Speaker: Prof. Keisuke Kaji, Professor of Biology of Reprogramming, Deputy Director of the Martin Lee Doctoral Scholarship Programme in Stem Cell & Regenerative Medicine
Topic: Molecular Mechanisms of Induced Pluripotent Cell (iPSC) Generation

Speaker: Dr. Kikuë TACHIBANA, Director, Department of Totipotency, Max Planck Institute of Biochemistry (MPIB), Martinsried, Germany
Topic: Mechanisms of transcriptional genome “awakening” and 3D chromatin reorganization at the start of life
Time: 4 PM (HKT)
CTSCB Stem Cell Seminar Series
Date: March 14, 2023
Abstract: How chromatin is reprogrammed to a totipotent “ground state” at the start of life remains a crucial question in biology. Totipotency is the developmental potential of a cell to give rise to all cell types and a whole organism. Reprogramming in early embryos is thought to occur by resetting of epigenetic modifications and activation of embryonic transcription in a process known as zygotic genome activation (ZGA). Despite the fundamental importance of ZGA for subsequent development, its essential regulators remain largely unknown in mammals. We recently identified the orphan nuclear receptor Nr5a2 as an essential transcription factor that activates the majority of ZGA genes in mouse 2-cell embryos. Genomic and biochemical data provide evidence that Nr5a2 has pioneering activity to open chromatin. We conclude that Nr5a2 is a key pioneer factor that transcriptionally “awakens” the genome during ZGA.
Early development is also accompanied by changes in genome architecture. There is mounting evidence that a mechanism of loop extrusion folds the genome into loops and topologically associating domains (TADs). Loop extrusion is halted at genomic sites bound by the zinc finger transcription factor CTCF, which is the main barrier to loop extrusion. By taking advantage of the oocyte-to-embryo transition, we discovered that the minichromosome maintenance complexes (MCMs) that function as replicative helicases during DNA replication impede loop extrusion in G1 phase. Therefore, 3D genome organization is shaped by loop extrusion and distinct types of barriers.

Speaker: Professor Johanna A. Joyce, University of Lausanne,Ludwig Institute for Cancer Research, Lausanne, Switzerland
Topic: Exploring and Therapeutically Exploiting the Tumor Microenvironment
Time: 5 PM (HKT)
CTSCB Stem Cell Seminar Series
Date: February 28, 2023
Abstract: Cancers develop in complex tissue environments, which they depend upon for sustained growth, invasion and metastasis. Different tissue and tumor microenvironments are populated by diverse cell types including innate and adaptive immune cells, fibroblasts, blood and lymphatic vascular networks, and specialized organ-specific cell types, which collectively have critical functions in regulating tumorigenesis. An example of an exquisitely organ-specific microenvironment is the brain, with a number of critical tissue-resident cells playing key roles in regulating brain cancer initiation, development and metastasis.
We explore how reciprocal communication between cancer cells and diverse immune and stromal cell types in the tumor microenvironment regulates each of the key stages of disease progression, as well as the response to therapeutic intervention. We then exploit this knowledge to devise novel and effective strategies to therapeutically target the tumor microenvironment, with a special emphasis on brain cancers.
Time: 9 AM – 6 PM (HKT)
Venue: INNO², 2/F, 17 Science Park West Avenue, The Hong Kong Science Park
Photo Album: Here
Explore more: www.ctscb-symposium.com

1st CTSCB Symposium on Stem Cell Translation
Date: February 9, 2023
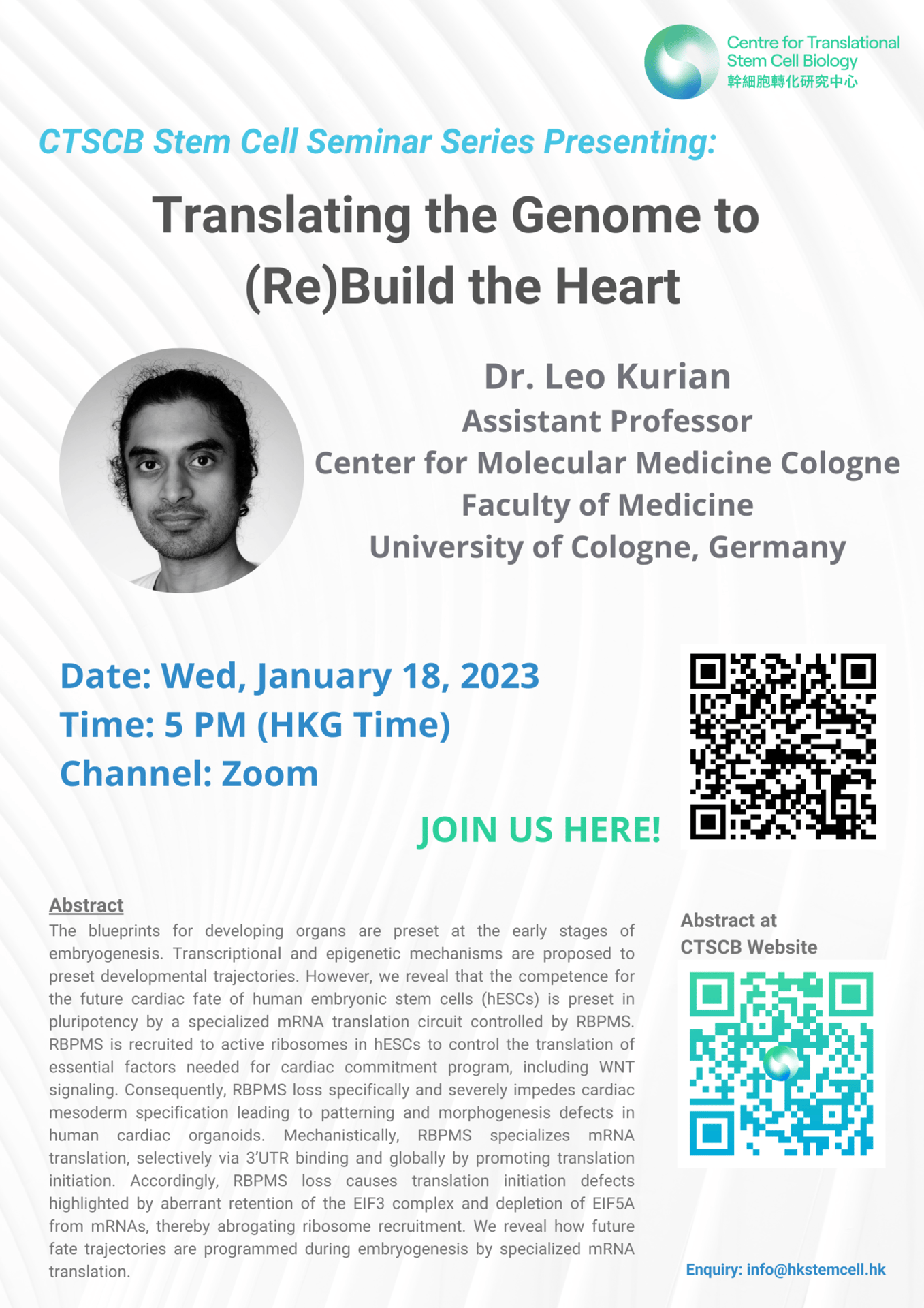
Abstract: The blueprints for developing organs are preset at the early stages of embryogenesis. Transcriptional and epigenetic mechanisms are proposed to preset developmental trajectories. However, we reveal that the competence for the future cardiac fate of human embryonic stem cells (hESCs) is preset in pluripotency by a specialized mRNA translation circuit controlled by RBPMS. RBPMS is recruited to active ribosomes in hESCs to control the translation of essential factors needed for cardiac commitment program, including WNT signaling. Consequently, RBPMS loss specifically and severely impedes cardiac mesoderm specification leading to patterning and morphogenesis defects in human cardiac organoids. Mechanistically, RBPMS specializes mRNA translation, selectively via 3’UTR binding and globally by promoting translation initiation. Accordingly, RBPMS loss causes translation initiation defects highlighted by aberrant retention of the EIF3 complex and depletion of EIF5A from mRNAs, thereby abrogating ribosome recruitment. We reveal how future fate trajectories are programmed during embryogenesis by specialized mRNA translation.
Speaker: Dr. Leo Kurian, Assistant Professor, Centre for Molecular Medicine Cologne, Faculty of Medicine, University of Cologne, Germany
Topic: Translating the Genome to (Re)Build the Heart
Time: 5 PM (HKT)
CTSCB Stem Cell Seminar Series
Date: January 18, 2023
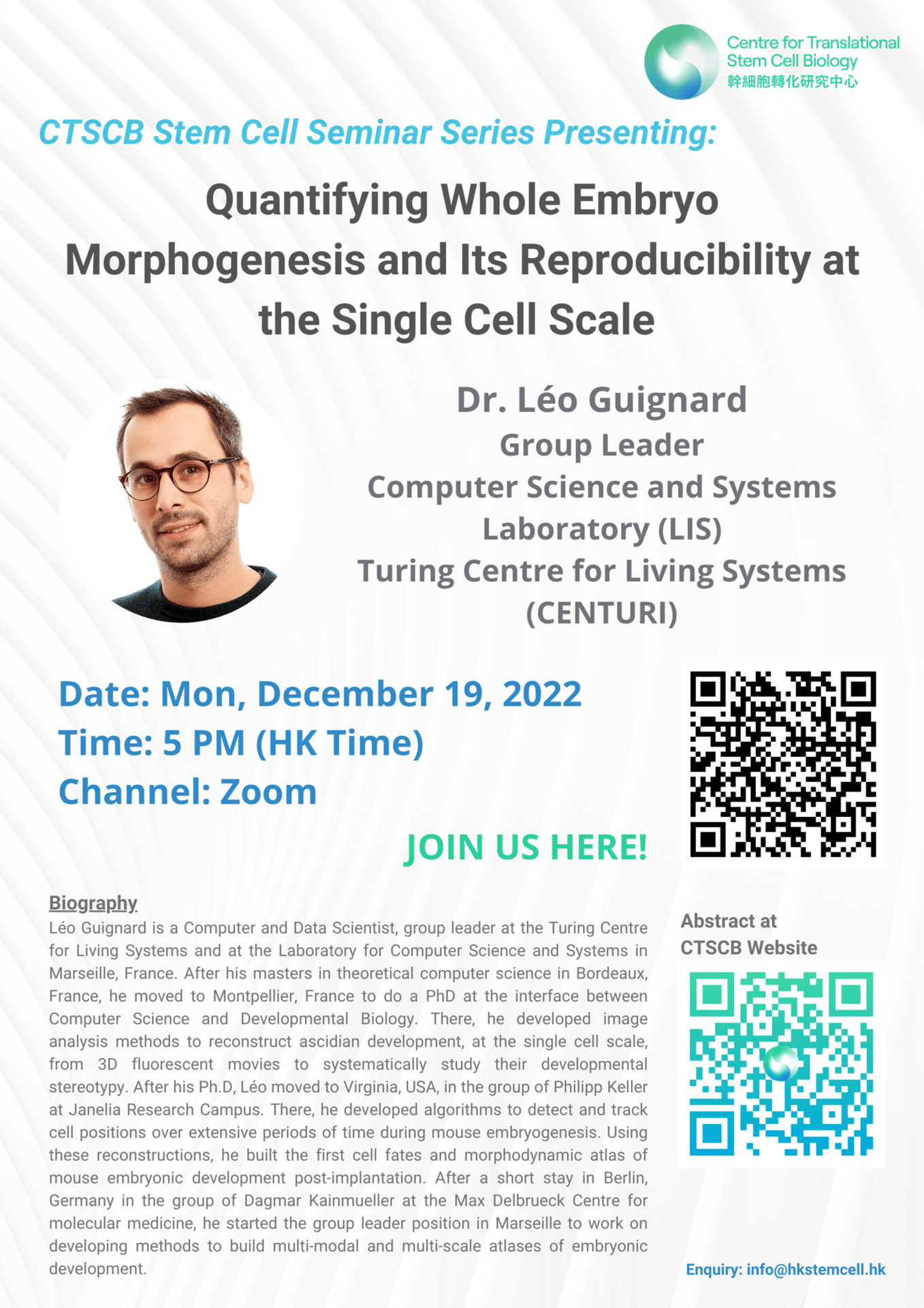
Abstract: Embryogenesis, the process of forming a functioning organism from a fertilised egg involves the precise and complex orchestration of hundreds to billions of cells dividing, changing their position, shape or size across hours, days or months. This orchestration often consists in a predefined set of instructions which in turn implies that within species, embryos follow the same developmental program and therefore are morphologically similar. Nonetheless, this reproducibility of the development can be observed at different scales between different species, from a reproducibility at the single cell scale to the tissue scale. Here, we will discuss the different scales of reproducibility and their impact on the development by looking at two species that exhibit different embryogenesis programs: mice and ascidians. We will show how we quantified embryogenesis reproducibility by building dynamic statistical atlases of the development of these two species, at the single cell scale, from 3D movies acquired by fluorescence microscopy. Finally, we will show how such quantitative single-cell atlases allowed us to give insights on the ascidian evolutionary dilemma.
Speaker: Dr. Leo Guignard, Group Leader, Computer Science and Systems Laboratory (LIS), Turing Centre for Living System (CENTURI)
Topic: Quantifying Whole Embryo Morphogenesis and Its Reproducibility at the Single Cell Sacle
Time: 5 PM (HKT)
CTSCB Stem Cell Seminar Series
Date: December 19, 2022
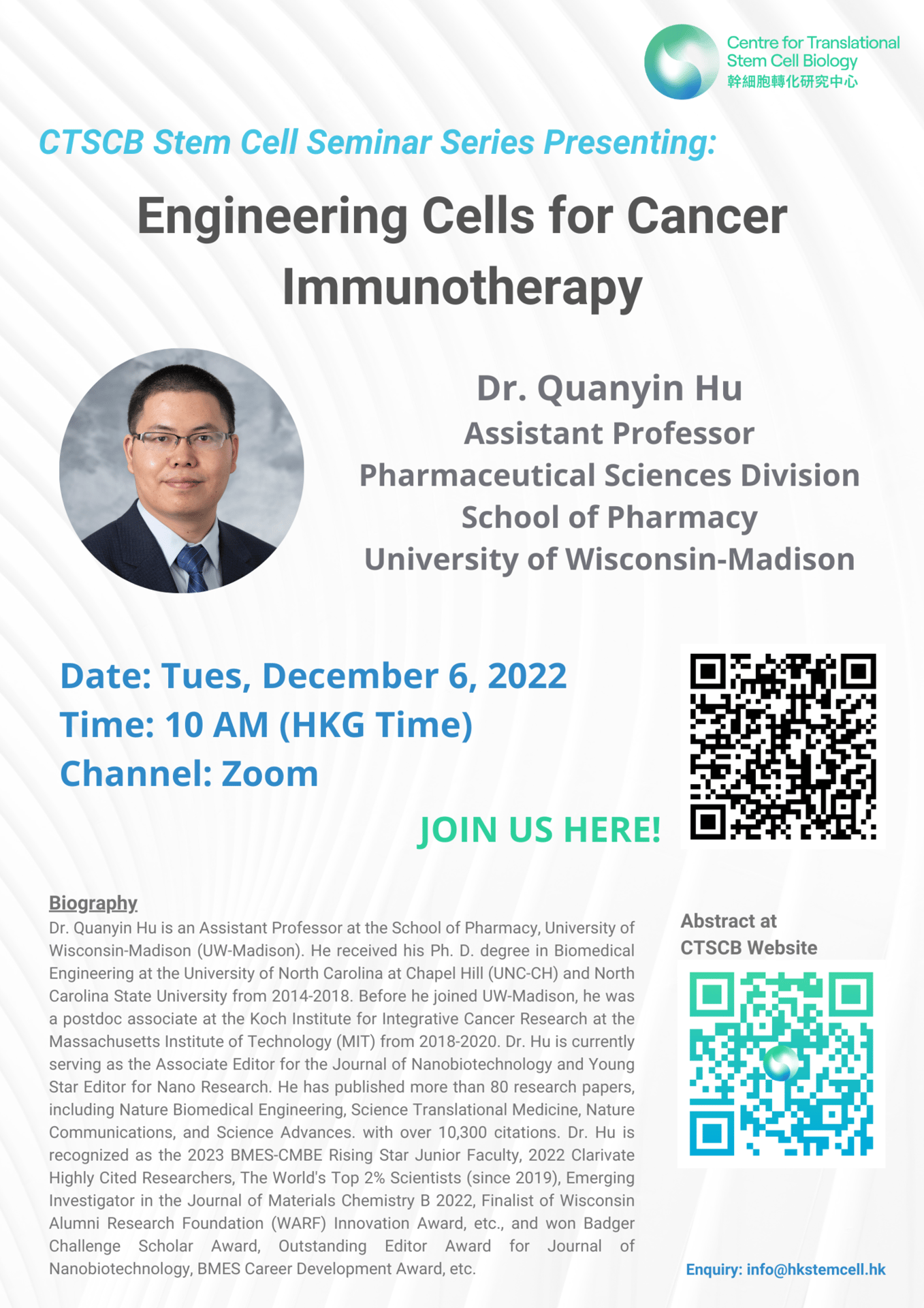
Abstract: Immunotherapy is leading a paradigm shift in the treatment of various diseases, including cancer. However, the limited objective response rate and side effects impede the clinical applications of immunotherapy. As important biological entities and natural carriers for proteins and molecules, cells with low immunogenicity and toxicity have attracted considerable attention for biomedical applications and have achieved encouraging progress, especially in tumor immunotherapy. The convergence of multiple disciplines has equipped cell engineering with control over their spatiotemporal distribution to enhance treatment efficacy and reduce side effects. In this talk, I will introduce our recent efforts in locoregionally engineering tumor-associated macrophages to CAR-Macrophages for post-surgery glioma treatment, and enhancing tumor cell pyroptosis by preventing ESCRT-mediated cell membrane repair with bacteria-based delivery systems.
Speaker: Dr. Quanyin Hu, Assistant Professor, Pharmaceutical Sciences Division, School of Pharmacy, University of Wisconsin-Madison
Topic: Engineering Cells for Cancer Immunotherapy
Time: 10 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: December 6, 2022
Abstract: X chromosome dosage compensation represents an epigenetic phenomenon where coordinated regulation of a whole chromosome is required to compensate the imbalance of X-linked gene dosage between the sexes. We study this process in placental mammals, where X chromosome dosage compensation occurs through X chromosome inactivation (XCI), which results in the formation and maintenance of the silent nuclear compartment of the inactive X-chromosome (Xi). XCI is an essential developmental process in which roughly a thousand genes are silenced by the non-coding RNA Xist and therefore offers the unique opportunity to understand mechanistically how RNA molecules can establish a distinct nuclear compartment. The female-specific mosaicism resulting from random inactivation of one of the two X-chromosomes impacts human health as it affects the outcome of X-linked diseases. Our recent advances in studying the complex interplay among Xist RNA, interacting proteins, chromatin and transcription will be presented. The talk will also address how a distinct X chromosome dosage compensation process is employed in early human development and our efforts towards developing unique treatments for X-linked diseases.
Speaker: Professor Kathrin Plath, Professor, Department of Biological Chemistry, David Geffen School of Medicine, University of California, Los Angeles
Topic: New Insights into the Function of IncRNAs and Mechanisms Underlying X-Chromosome Deosage Compensation
Time: 11 AM (HKT)
CTSCB-HKUST Joint Seminar
Date: November 30, 2022

Abstract: Over the last two decades my lab has increasingly focused on the mechanisms whereby blood stem cells are subverted to cause haematological malignancies, using human myeloproliferative neoplasms (MPNs) as a tractable model.
In work which transformed MPN diagnosis and catalysed development of therapeutic JAK inhibitors, we and others identified phenotypic driver mutations in JAK2 and CALR which activate the JAK/STAT pathway and are present in most MPN patients. We have subsequently employed a variety of approaches to describe the biological consequences of these mutations at molecular, cellular and organismal levels. In addition to altering clinical practice, our studies have led to unexpected insights into cancer biology as well as normal cytokine signalling.
Recent highlights include: (i) first demonstration in any cancer that mutation order affects stem and progenitor behaviour, thus influencing clinical presentation, disease outcome and response to therapy (Ortmann et al NEJM 2015); (ii) description of unexpected non-canonical mechanisms of JAK/STAT signalling (Dawson et al Nature 2009; Dawson et al Cell Reports 2013); Park et al EMBO J 2016); (iii) a new MPN classification based on causal biological mechanisms and the development of personalised predictions tailored to individual patients (Grinfeld et al NEJM 2018); (iv) the use of somatic mutations as clonal markers to reconstruct the cellular ancestry of human haematopoiesis (Lee-Six et al Nature 2018) and show that MPN driver mutations frequently arise in early childhood or in utero (Williams et al Nature 2022).
Speaker: Professor Tony Green, Professor of Haemato-Oncology, Wellcome-MRC Cambridge Stem Cell Institute, Department of Haematology, University of Cambridge
Topic: JAK/STAT Signalling, Stem Cell Subversion and Blood Cancers
Time: 10:30 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: November 25, 2022
Abstract: Spatial omics aim to survey the natural state of cells in native tissues, to identify the location-defined cell fates and to understand how the cells are communicating within their community. The spatial transcriptomic analysis with the integration of single-cell RNA-seq, imaging reconstruction and tracing will allow for studying tissue heterogeneity at different scales, dissecting new layers of molecular connectivity between the genome and its functional output, and leading to many insightful discoveries during embryo development and tissue injury.
Speaker: Professor Guangdun Peng, Professor and Principal Investigator at Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences
Topic: Spatial Omics Analysis for Tissue Development and Injury
Time: 4 PM (HKT)
CTSCB Stem Cell Seminar Series
Date: November 15, 2022

Biography: Work by Dr. Sonja Schrepfer is at the forefront of stem immunobiology and paves the way for treatment of a wide range of diseases – from supporting functional recovery of failing myocardium to the derivation of other cell types to treat diabetes, blindness, cancer, lung, neurodegenerative, and related diseases. She spent many years examining in detail the fetomaternal interface for application to the envisioned cell therapy. Her work demonstrates that hypo-immune cells reliably evade immune rejection in allogeneic recipients from various species, and further, these cells show long-term survival without immunosuppression. These findings - truly hypo-immunogeneic iPSCs - achieve the “holy grail” of stem cell immunobiology and have been have been highlighted in leading journals such as Nature and Science and by numerous prestigious awards.
Dr. Schrepfer is Professor at the University of California San Francisco (UCSF), and Scientific Founder and SVP from Sana Biotechnology, Inc.
Speaker: Professor Sonja Schrepfer, Professor of Surgery at Transplant and Stem Cell Immunobiology Laboratory, University of California San Francisco
Topic: Unleashing Cells from Immune Rejection to Make Off-The-Shelf Therapies
Time: 11 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: November 9, 2022
Abstract: Understanding lineage specification during human pre-implantation development is a key requirement to improve assisted reproductive technologies and stem cell research. Single-cell RNAseq has allowed insights into the processes orchestrating human pre-implantation development. However, the exact sequence of molecular events leading to cell fate specification remains to be defined. Here, we employ pseudotime analysis of scRNAseq data to reconstruct the developmental progress of early mouse and human embryo development. Using time-lapse annotated embryos, we provide an integrated analysis allowing precise ordering of continuous transcriptomic changes throughout human development. Our analysis revealed that human trophectoderm/inner cell mass transcriptomes diverge at the transition from the B2 to B3 blastocyst stage, just before blastocyst expansion. Moreover, we explore the dynamics of fate markers IFI16 and GATA4 and show that they gradually become mutually exclusive upon establishment of epiblast and primitive endoderm fates, respectively. We also provide evidence that NR2F2 marks trophectoderm maturation initiating from the polar side, and subsequently spreads to all cells after implantation. Altogether, our study pinpoints the precise timing of lineage specification events in the human embryo and identifies transcriptomic hallmarks and cell fate markers.
I will highlight how we used this knowledge of human embryos to benchmark existing stem cell models, including integrated models such as blastoids.
Speaker: Dr. Laurent David, Group Leader of Centre for Translational Immunology, Associate Professor in Cell Biology, Nantes Université
Topic: Studying Human Peri-Implantation Development with Embryos and Stem Cell Models
Time: 4 PM (HKT)
CTSCB Stem Cell Seminar Series
Date: October 5, 2022

Abstract: Harnessing the power of the immune system to treat cancer has proven to be a promising therapeutic option for patients. The overall goal of Dr. Rafiq's research is to use mechanistic insight of immune effector cell function and interaction with cancer cells in the tumor microenvironment to inform the development and translation of novel engineered cellular immunotherapies. In particular, her work focuses on the genetic engineering of synthetic receptors known as Chimeric Antigen Receptors (CAR). Despite the transformative effect CAR T cells have had on the treatment of some types of leukemias and lymphoma in recent years, obstacles remain in expanding this technology for more widespread use in cancer. As a translational cancer researcher, Dr. Rafiq's studies aim to engineer novel CARs to target both solid and hematological malignancies as well as deliver immune-modulating or toxic agents locally to the tumor microenvironment.
Speaker: Dr. Sawrish Rafiq, Assistant Professor of Department of Hematology and Medical Oncology, Emory University School of Medicine
Topic: Engineering Functionally Improved CAR T Cells for Hematological and Solid Tumors
Time: 9 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: September 27, 2022
Abstract: The Regenerative Medicine of the future will rely on being able to produce a wide variety of “designer” tissues of choice (neurons, heart cells, liver cells, etc) that can be used for Personalised tissue replacement in the clinic. This dream has become achievable thanks to the Nobel Prize winning discovery 16 years ago of human induced Pluripotent Stem (iPS) cells, a revolutionary technology allowing any cells in our body to be converted into pluripotent stem cells from which almost any desired target tissue of choice could be derived by differentiation in vitro.
However, key challenges have to be overcome before the promise of stem cell therapeutics becomes a reality. First, we do not yet know how to control with precision the differentiation of iPS cells into the target tissues of choice. Most procedures to induce differentiation of iPS cells remain quite inefficient, unspecific, and unsafe (i.e. some of the cells obtained after differentiation retain the capacity to keep proliferating and make tumours, for reasons that are not understood). Secondly, even when generated in exactly the same way in the laboratory, iPS cells derived from some people can be programmed better into target tissues than those from other people, for reasons that remain unknown.
In this talk Professor Carazo Salas will describe multicolour, multiday high-content microscopy phenomics pipelines that his group has recently established that enable to visualise and predict the dynamical cell fate changes of human Pluripotent Stem Cells (hPSCs) ‘live’, collectively and at single-cell level. He will describe the integrated experimental and computational approaches they have developed to make this possible, including novel ‘live’ reporters of cell fate and multi-reporter hPSC lines generated by CRISPR/Cas9 allowing multiplexed monitoring of proliferation and fate dynamics as well as deep learning-enhanced morphological phenotyping methods, and briefly exemplify the biological discoveries they are enabling. Mapping the complex spatiotemporal dynamics of human stem cells as they proliferate and make cell fate decisions will be key in the future to improve our understanding of how to robustly engineer differentiated tissues for therapeutic applications.
Speaker: Professor Rafael E. Carazo Salas, Professor and Chair of Biomedical Sciences, University of Bristol
Topic: Predicting 'Live' Cell Fate Dynamics in hPSCs by Deep Learning-Enhanced Morphological Profiling
Time: 5 PM (HKT)
CTSCB Stem Cell Seminar Series
Date: September 14, 2022
Abstract: The adoptive transfer of T cells expressing chimeric antigen receptors (CARs) has demonstrated clinical efficacy in the treatment of advanced cancers, with anti-CD19 CAR-T cells achieving up to 90% complete remission among patients with relapsed B-cell malignancies. However, challenges such as antigen escape and immunosuppression limit the long-term efficacy of adoptive T-cell therapy. Here, I will discuss the development of and clinical data on next-generation T cells that can target multiple cancer antigens and resist antigen escape. I will also present recent work on tuning CAR signaling activities via rational protein design to achieve greater in vivo anti-tumor efficacy. This presentation will highlight the potential of synthetic biology in generating novel mammalian cell systems with multifunctional outputs for therapeutic applications.
Speaker: Dr. Yvonne Chen, Associate Professor, Department of Microbiology, Immunology & Molecular Genetics, University of California, Los Angeles
Topic: Engineering Nex-Generation T Cells for Cancer Immunotherapy
Time: 10 AM (HKT)
CTSCB-HKUST Joint Seminar
Date: August 24, 2022

Abstract: Recent years have seen the rapid growth of big data in immunology and immune-oncology research. However, leveraging the vast amount of public data resources to make new findings is still challenging for most immunologists due to the complexity and heterogeneity of published datasets. I will introduce two data-integrative frameworks developed to help immunologists to understand intercellular signaling mechanisms, with applications in studying cancer immunotherapy resistance. The first framework CytoSig (https://cytosig.ccr.cancer.gov) contains a vast amount of cytokine treatment response data curated from public repositories. CytoSig can reliably predict cytokine signaling cascades in human inflammatory diseases and cancers. The second framework Tres (https://resilience.ccr.cancer.gov) provides many T-cell genomics datasets and interactive functions for immune oncologists to study molecular markers of T-cell anti-tumor efficacies. The Tres model also identified FIBP knockout as a new approach to potentiate cellular immunotherapies in solid tumors.
Speaker: Dr. Peng Jiang, Earl Stadtman Investigator - National Cancer Institute at National Institutes of Health
Topic: Big Data Approaches to Study Intercellular Signaling in Cancer Immunotherapy Resistance
Time: 10 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: June 28, 2022
Abstract: The process of implantation and the cellular interactions at the embryo-maternal interface are intrinsically difficult to analyse, as the implanting embryo is concealed by the uterine tissues. To examine the mechanisms mediating the interconnection of the embryo and the mother, we established a 3D biomimetic culture environment that harbours the key features of the implantation niche. This allowed direct analysis of trophoblast invasion and revealed the first embryonic interactions with the maternal vasculature. We found that implantation is mediated by the collective migration of penetrating strands of trophoblast giant cells, which acquire the expression of vascular receptors, ligands, and adhesion molecules, assembling a network for communication with the maternal blood vessels.
Speaker: Dr. Ivan Bedshov, Principal Investigator - Max Planck Institute for Molecular Biomoedicine Muenster
Topic: Cellular Mechanisms of Embryonic Development and Embryo-Maternal Interactions during Implantation
Time: 4 PM (HKT)
CTSCB Stem Cell Seminar Series
Date: June 14, 2022
Abstract: Old age is a major risk factor for many human diseases. Sporadic Alzheimer’s Disease (AD) represents a prime example, as it exclusively affects people at old age. Sporadic AD represents the overwhelming majority of all cases, and familial genetically defined early-onset cases are rare. Still, most research on AD has been performed on genetic causes and their directly related pathways, also because we were in lack of models that can reflect complex human genetics, physiology, and age in an appropriate human neuronal context. While patient-specific iPSC-based models represent an attractive solution, iPSC reprogramming results in cellular rejuvenation and thus yields phenotypically young neurons. By contrast, direct conversion of old patient fibroblasts into induced neurons (iNs) preserves endogenous signatures of aging. To control for the involvement of aging in human neuronal models for AD, we combined both technologies and generated age-equivalent fibroblast-derived iNs, as well as rejuvenated iPSC-derived neurons from a large cohort of AD patients and controls. In addition to their rejuvenated state, we found that iPSC neurons transcriptionally resemble prenatal developmental stages, while iNs reflect old epigenetic ages, adult-like transcriptome stages, and show little correlation with the prenatal brain. Thus, not surprisingly, only age-equivalent adult-like iNs, but not rejuvenated prenatal-like iPSC neurons, revealed strong AD patient-specific signatures that revealed high concordance with bulk and single cell transcriptome data from earlier post-mortem studies. Longitudinal identity and cellular ontogenesis mapping revealed that AD iNs reflect a hypo-mature neuronal identity characterized by markers of stress, cell cycle, glycolytic reprogramming, and de-differentiation, and which share similarities with malignant cancer cell transformation and age-dependent epigenetic erosion. Pathological isoform switching of the glycolytic enzyme pyruvate kinase (PKM) towards the cancer-associated PKM2 isoform conferred a large proportion of the transcriptional and metabolic alterations in AD iNs, both via metabolic malfunctions and via nuclear translocation. Our patient-based iN model thus identifies AD-related neuronal changes as part of an active cellular program that impairs neuronal cell identity and neuronal resilience by utilizing cancer-related patho-mechanisms.
Speaker: Dr. Jerome Mertens, Assistant Professor and Head of Neural Aging Laboratory - University of Innsbruck
Topic: Neuronal Fate Loss and Metabolic Transformation in Age-Equivalent Alzheimer Patient Neurons
Time: 4 PM (HKT)
CTSCB-HKUST Joint Seminar
Date: May 27, 2022
Abstract: Reconstructing the circuits that control how cells adopt specific fates and engineering these circuits to reprogram cellular functions are major challenges in biology. I will introduce a series of experimental and computational frameworks such as “Waddington-OT”, “Raman2RNA” for reconstructing molecular dynamics over time and in live cells through single-cell genomics and imaging. I will introduce how we can use these approaches to decode the cellular and molecular mechanisms governing reprogramming and development.
Speaker: Dr. Jian Shu, Assistant Professor - Harvard Medical School & Massachusetts General Hospital, Associate Member at Broad Institute of MIT and Harvard
Topic: Decoding and Engineering Cell Fates and Circuits
Time: 9 AM (HKT)
CTSCB-HKUST Joint Seminar
Date: May 20, 2022
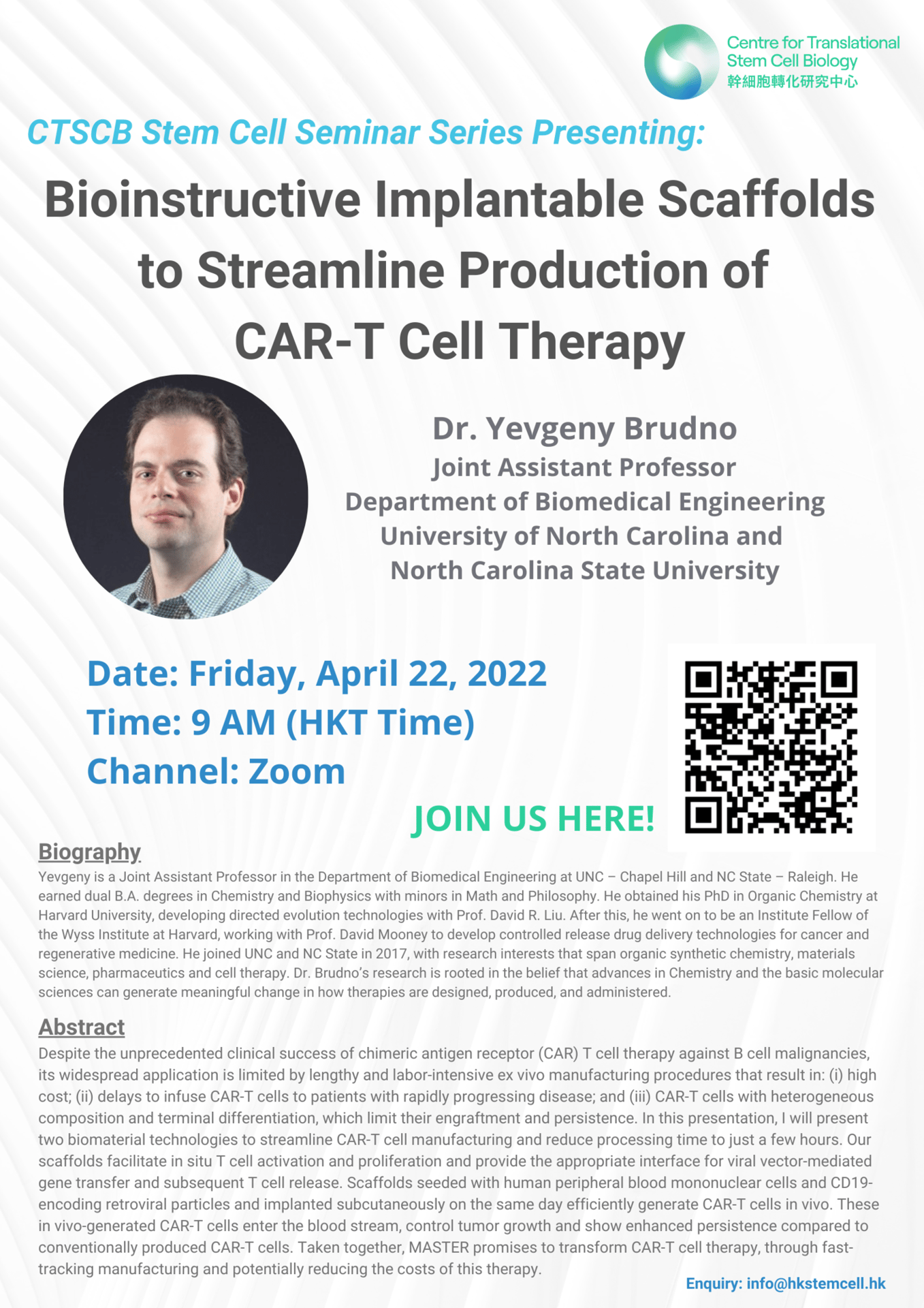
Abstract: Despite the unprecedented clinical success of chimeric antigen receptor (CAR) T cell therapy against B cell malignancies, its widespread application is limited by lengthy and labor-intensive ex vivo manufacturing procedures that result in: (i) high cost; (ii) delays to infuse CAR-T cells to patients with rapidly progressing disease; and (iii) CAR-T cells with heterogeneous composition and terminal differentiation, which limit their engraftment and persistence. In this presentation, I will present two biomaterial technologies to streamline CAR-T cell manufacturing and reduce processing time to just a few hours. Our scaffolds facilitate in situ T cell activation and proliferation and provide the appropriate interface for viral vector-mediated gene transfer and subsequent T cell release. Scaffolds seeded with human peripheral blood mononuclear cells and CD19-encoding retroviral particles and implanted subcutaneously on the same day efficiently generate CAR-T cells in vivo. These in vivo-generated CAR-T cells enter the blood stream, control tumor growth and show enhanced persistence compared to conventionally produced CAR-T cells. Taken together, MASTER promises to transform CAR-T cell therapy, through fast-tracking manufacturing and potentially reducing the costs of this therapy.
Speaker: Dr. Yevgeny Brudno, Joint Assistant Professor at Department of Biomedical Engineering - University of North Carolina and North Carolina State University
Topic: Bioinstructive Implantable Scaffolds to Streamline Production of CAR-T Cell Therapy
Time: 9 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: April 22, 2022

Abstract: Recent advances in single-cell technologies have elucidated the transcriptional heterogeneity in cancer cells and has enabled identification of sub-populations in tumors. However, the functional consequences of this transcriptional heterogeneity remain unknown. Do distinct transcriptional units correspond to functional differences in tumor cells? And can we identify and predict immature tumor initiating cells that have been shown to drive tumor growth and metastasis? We performed single cell RNA sequencing in breast cancer patient samples and developed a novel computational tool, CytoTRACE that can agnostically determine immature cell populations across different tissues, organisms, and diseased states. By using a combination of single-cell data, CytoTRACE, bulk tumor deconvolution, patient derived xenografts and lineage tracing we have identified distinct populations of cells in breast cancer responsible for tumor growth and metastasis. We have found that the hematopoietic transcriptional adaptor and T-cell leukemia oncogene, LMO2, is critical for early metastatic dissemination. Our work is now focused on the molecular signaling that maintains/drives transitions of distinct cell populations and the factors in the microenvironment that influence these transitions.
Speaker: Dr. Shaheen Sikandar, Assistant Professor of MCD Biology - The University of California, Santa Cruz
Topic: Elucidating Functional Heterogeneity in Breast Cancer Cells
Time: 9 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: March 25, 2022
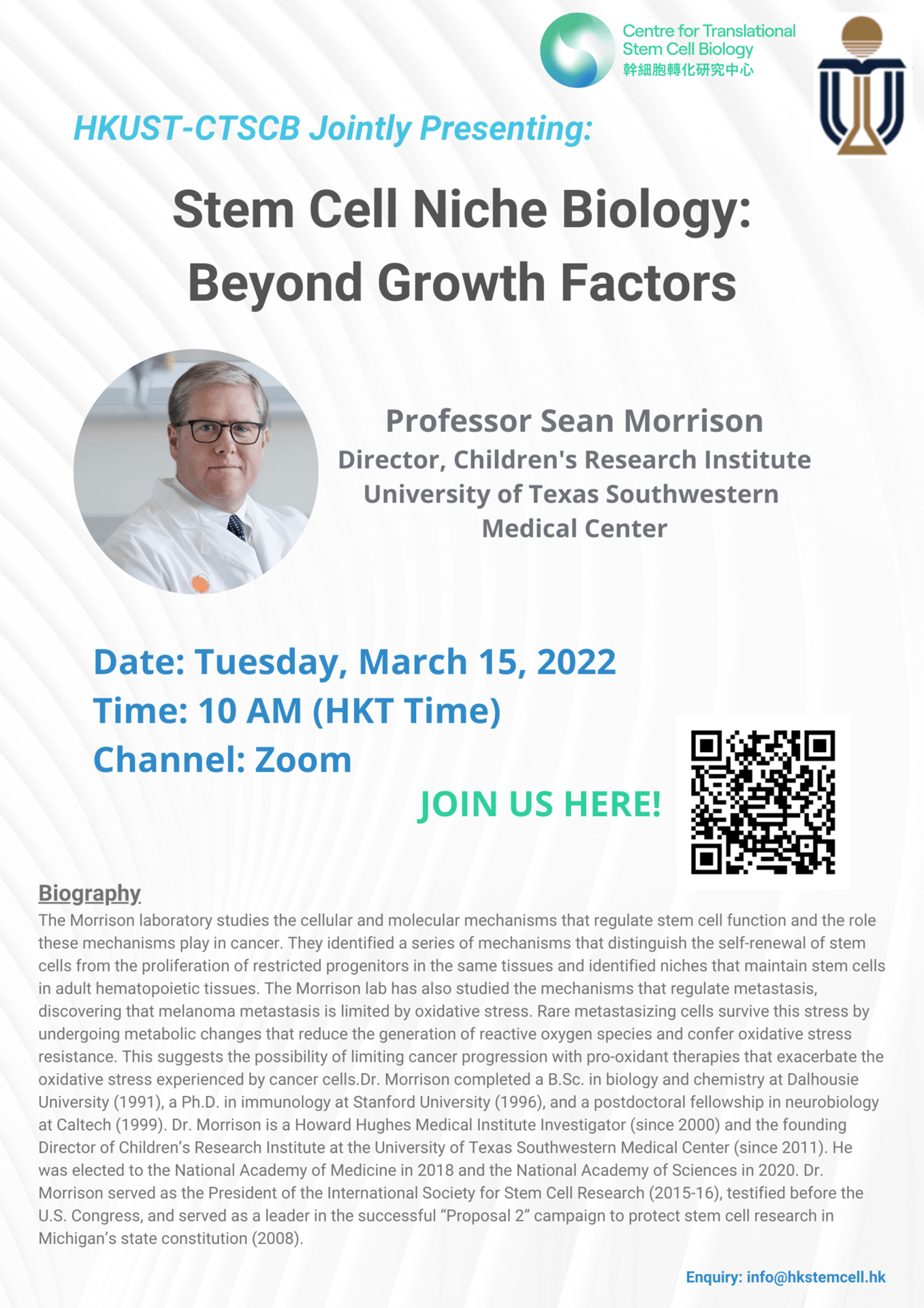
Abstract: The Morrison laboratory studies the cellular and molecular mechanisms that regulate stem cell function and the role these mechanisms play in cancer. They identified a series of mechanisms that distinguish the self-renewal of stem cells from the proliferation of restricted progenitors in the same tissues and identified niches that maintain stem cells in adult hematopoietic tissues. The Morrison lab has also studied the mechanisms that regulate metastasis, discovering that melanoma metastasis is limited by oxidative stress. Rare metastasizing cells survive this stress by undergoing metabolic changes that reduce the generation of reactive oxygen species and confer oxidative stress resistance. This suggests the possibility of limiting cancer progression with pro-oxidant therapies that exacerbate the oxidative stress experienced by cancer cells. Dr. Morrison completed a B.Sc. in biology and chemistry at Dalhousie University (1991), a Ph.D. in immunology at Stanford University (1996), and a postdoctoral fellowship in neurobiology at Caltech (1999). Dr. Morrison is a Howard Hughes Medical Institute Investigator (since 2000) and the founding Director of Children’s Research Institute at the University of Texas Southwestern Medical Center (since 2011). He was elected to the National Academy of Medicine in 2018 and the National Academy of Sciences in 2020. Dr. Morrison served as the President of the International Society for Stem Cell Research (2015-16), testified before the U.S. Congress, and served as a leader in the successful “Proposal 2” campaign to protect stem cell research in Michigan’s state constitution (2008).
Speaker: Professor Sean Morrison, Director - Children's Research Institute at University of Texas Southewestern Medical Center
Topic: Stem Cell Niche Bioology: Beyond Growth Factors
Time: 10 AM (HKT)
CTSCB-HKUST Joint Seminar
Date: March 15, 2022
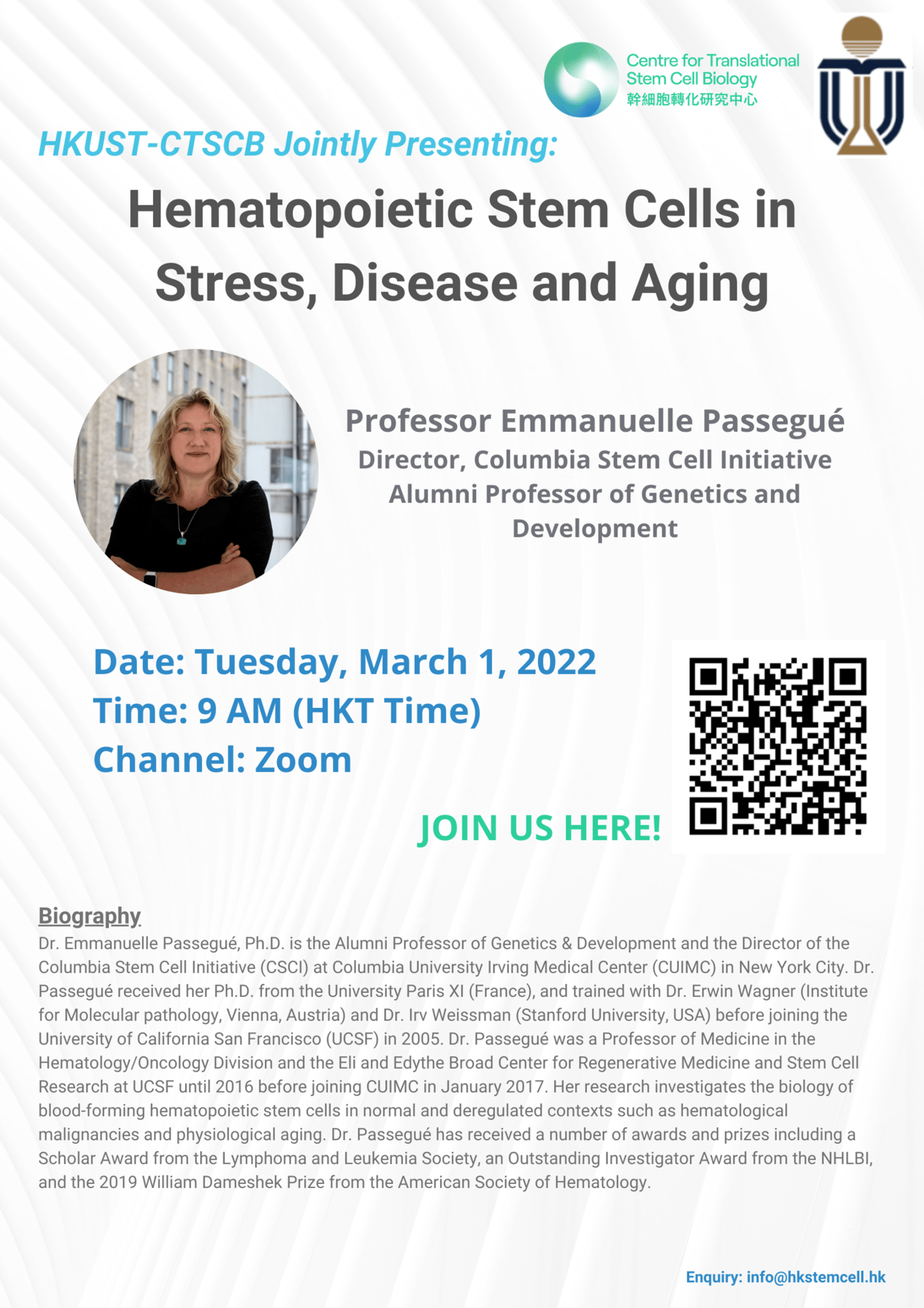
Abstract: The process of implantation and the cellular interactions at the embryo-maternal interface are intrinsically difficult to analyse, as the implanting embryo is concealed by the uterine tissues. To examine the mechanisms mediating the interconnection of the embryo and the mother, we established a 3D biomimetic culture environment that harbours the key features of the implantation niche. This allowed direct analysis of trophoblast invasion and revealed the first embryonic interactions with the maternal vasculature. We found that implantation is mediated by the collective migration of penetrating strands of trophoblast giant cells, which acquire the expression of vascular receptors, ligands, and adhesion molecules, assembling a network for communication with the maternal blood vessels.
Speaker: Professor Emmanuelle Passegue, Director - Columbia Stem Cell Initiative; Alumni Professor of Genetics and Development
Topic: Hematopoietic Stem Cells in Stress, Disease
Time: 9 AM (HKT)
CTSCB-HKUST Joint Seminar
Date: March 1, 2022
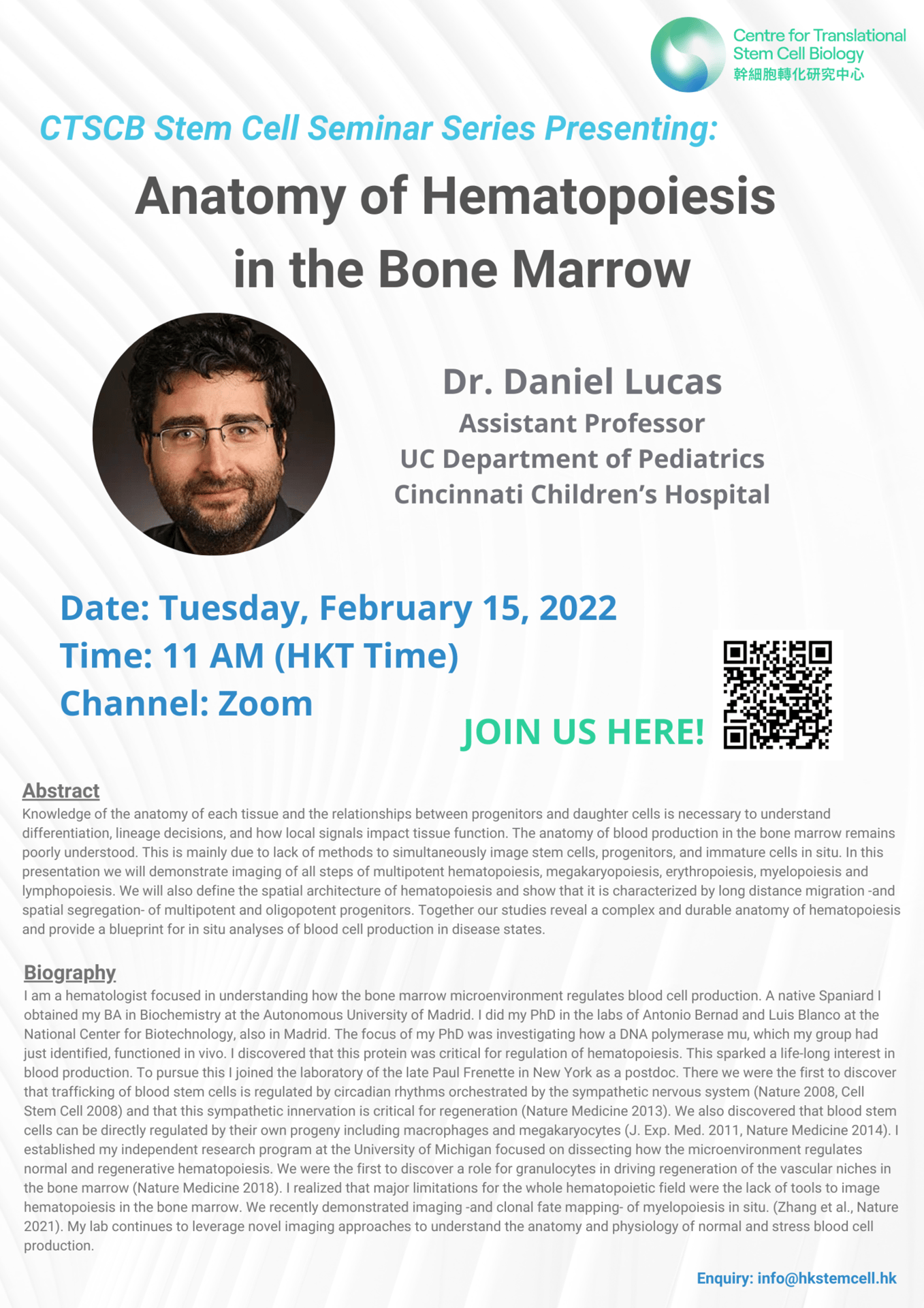
Abstract: The process of implantation and the cellular interactions at the embryo-maternal interface are intrinsically difficult to analyse, as the implanting embryo is concealed by the uterine tissues. To examine the mechanisms mediating the interconnection of the embryo and the mother, we established a 3D biomimetic culture environment that harbours the key features of the implantation niche. This allowed direct analysis of trophoblast invasion and revealed the first embryonic interactions with the maternal vasculature. We found that implantation is mediated by the collective migration of penetrating strands of trophoblast giant cells, which acquire the expression of vascular receptors, ligands, and adhesion molecules, assembling a network for communication with the maternal blood vessels.
Speaker: Dr. Daniel Lucas, Assistant Professor - UC Department of Pediatrics Clincinnati Children's Hospital
Topic: Anatomy of Hematopoiesis in the Bone Marrow
Time: 11 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: Feb 15, 2022

Abstract: The process of implantation and the cellular interactions at the embryo-maternal interface are intrinsically difficult to analyse, as the implanting embryo is concealed by the uterine tissues. To examine the mechanisms mediating the interconnection of the embryo and the mother, we established a 3D biomimetic culture environment that harbours the key features of the implantation niche. This allowed direct analysis of trophoblast invasion and revealed the first embryonic interactions with the maternal vasculature. We found that implantation is mediated by the collective migration of penetrating strands of trophoblast giant cells, which acquire the expression of vascular receptors, ligands, and adhesion molecules, assembling a network for communication with the maternal blood vessels.
Speaker: Dr. Ankur Sharma, Head of Onco-Fetal Ecosystem Laboratory - Harry Perkins Institute of Medical Research & Curtin University
Topic: Oncofetal ecosystem in HCC: Spatial localisation and clinical implications
Time: 11 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: Jan 18, 2022

Job Fair
Time: 12 NN
Venue: Unit 1105, 11/F, 17W Science Park West Avenue,
HKSTP, Shatin, N.T.
Brief Intro: Centre for Translational Stem Cell Biology is going to hold a soft-opening on….
Link: http://www.linkedin.com/in/ctscbhk
Full detail: Centre for Translational Stem Cell Biology is going to hold a soft opening on January 14, 2022 to celebrate the establishment. Our Managing Director, Prof. Pengtao Liu, cordially invites everyone of you to join us on the big day. Refreshment will be provided. Click the link to register!
Date: January 14, 2022

Abstract: The process of implantation and the cellular interactions at the embryo-maternal interface are intrinsically difficult to analyse, as the implanting embryo is concealed by the uterine tissues. To examine the mechanisms mediating the interconnection of the embryo and the mother, we established a 3D biomimetic culture environment that harbours the key features of the implantation niche. This allowed direct analysis of trophoblast invasion and revealed the first embryonic interactions with the maternal vasculature. We found that implantation is mediated by the collective migration of penetrating strands of trophoblast giant cells, which acquire the expression of vascular receptors, ligands, and adhesion molecules, assembling a network for communication with the maternal blood vessels.
Speaker: Dr. Andy Tay, Presidential Yong Professor - National University of Singapore
Topic: Overcoming Hurdles in Immune-cell Engineering with Nanotechnology
Time: 9 AM (HKT)
CTSCB Stem Cell Seminar Series
Date: Dec 7, 2021
Copyright ©2024 All rights reserved | by Centre for Translational Stem Cell Biology